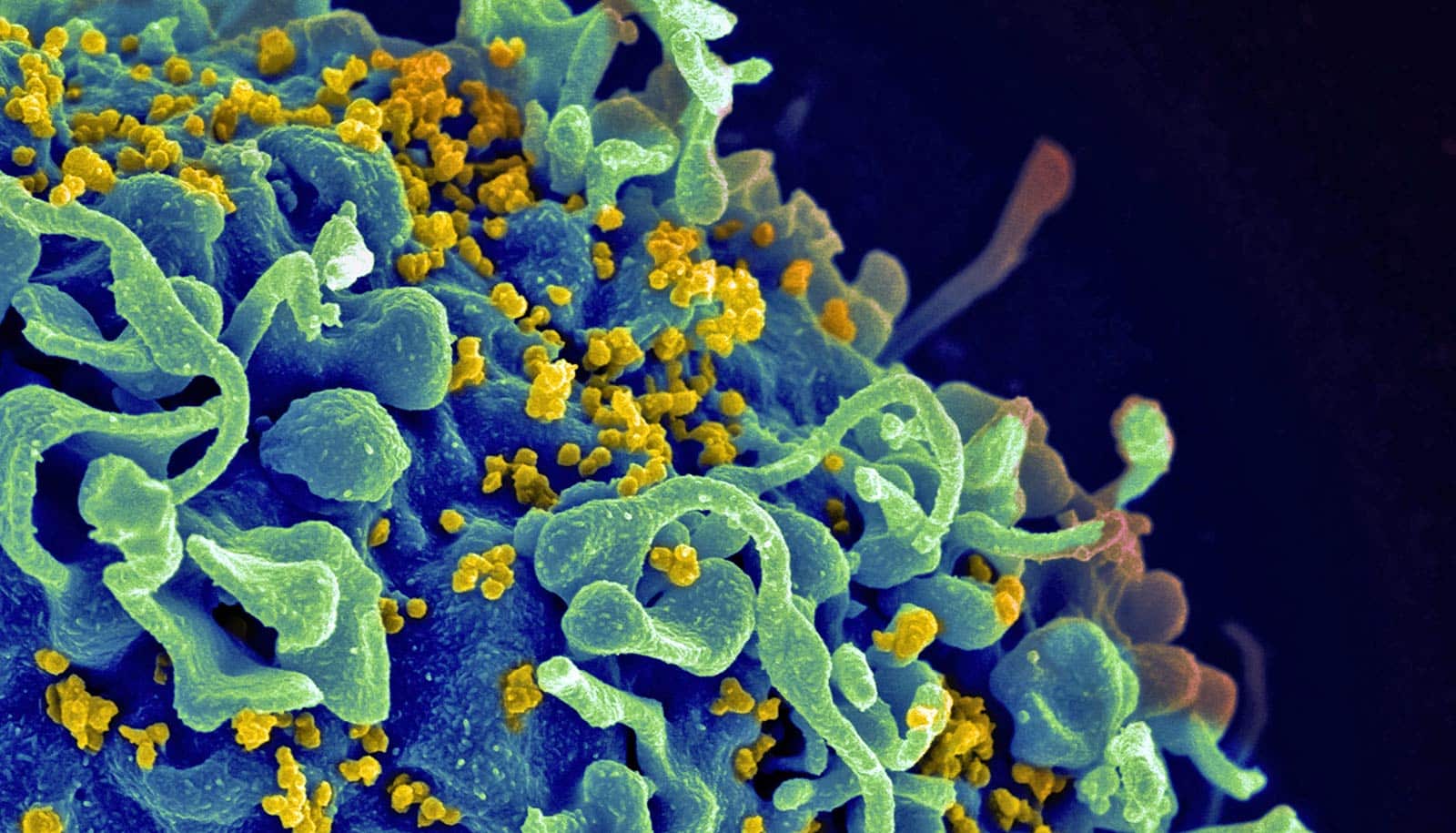
A potential vaccine component has led to strong protection against HIV in primates, a new study shows.
To block infection from HIV, a successful vaccine will require a combination of ingredients, including at least three antibody targets and a substance that boosts immune responses.
The new study takes a step toward achieving that goal.
The new component elicits an antibody that binds to part of HIV’s outer envelope, the researchers report.
“This is significant progress toward a viable HIV vaccine,” says Barton Haynes, director of the Duke University Human Vaccine Institute (DHVI) and senior author of the study in the journal Science Translational Medicine. “While we know this will be a many-step process, each step is an advancement toward our goal.”
Haynes and colleagues, including first author Kevin Saunders, director of research at DHVI, isolated neutralizing antibodies that arose from a person living with HIV.
These antibodies bind to a known site on the virus that is conserved among many HIV strains. Exploiting this interaction, the researchers built an immunogen that stimulates the protective neutralizing antibodies against the immunizing strain.
Along with the immunogen, the researchers tested potential adjuvants, which boost the immune response. The team found that the best adjuvant ingredient was a Toll-like receptor called TLR7/8 that is a common ingredient in other vaccines.
Together, the protein and adjuvant in the vaccine provided protection from infection.
“Our study supports the notion that relatively high levels of multiple broadly neutralizing antibody types will need to be induced by vaccination to prevent HIV infection,” Haynes says. “Our study also suggests that the use of the TLR7/8 agonist was key for inducing high concentrations of neutralizing antibodies and potently activating the immune system.”
The National Institutes of Health Division of AIDS and the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery funded the work.
The adjuvant used in this study, 3M-052-Alum, was developed by 3M and formulated by the Access to Advanced Health Institute.
Source: Duke University