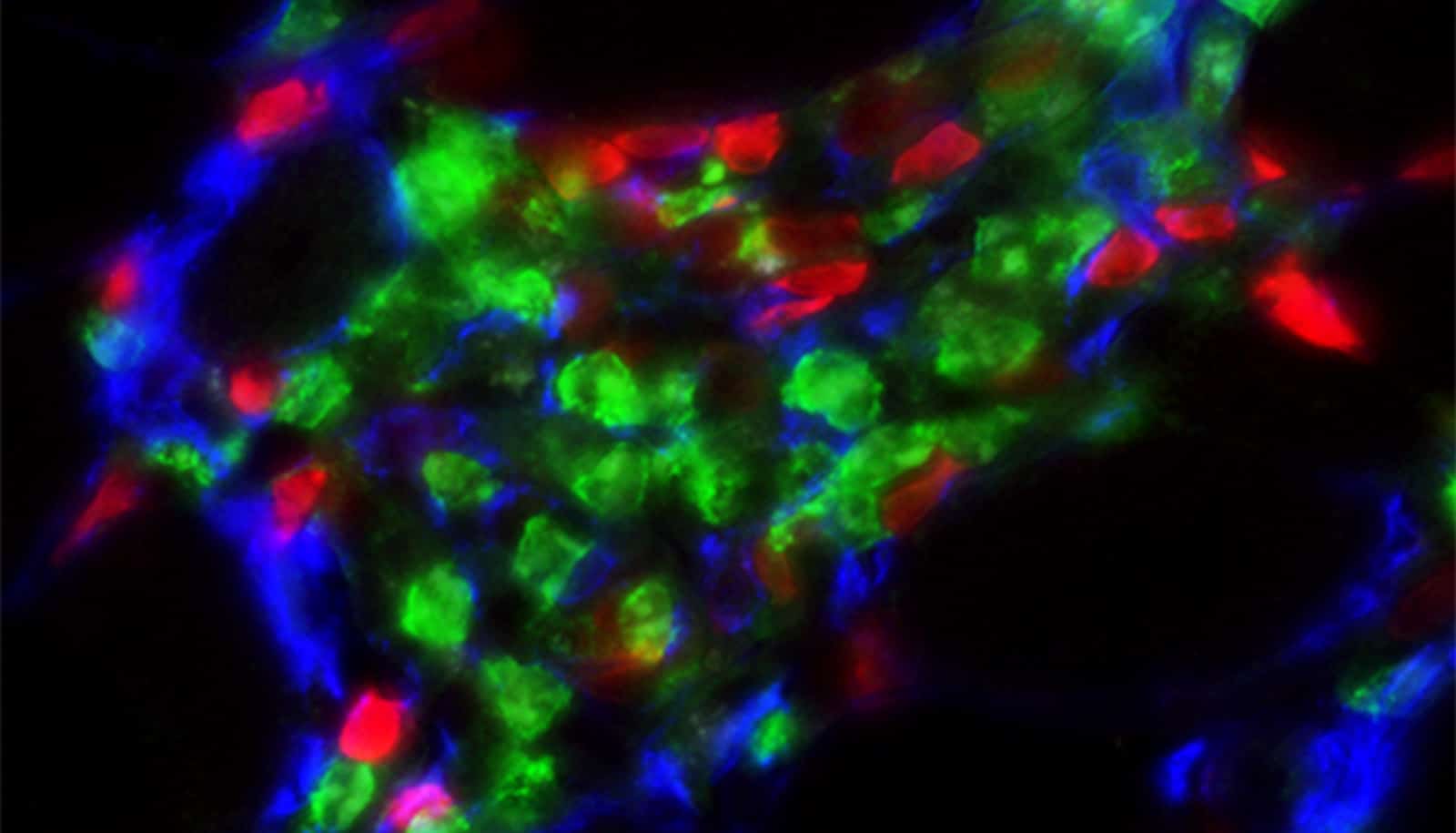
A new study reveals how chronic inflammation promotes muscle fibrosis.
The findings could lead to new therapies for people with Duchenne muscular dystrophy (DMD), a fatal muscle disease.
Chronic inflammation is a major pathological process contributing to the progression and severity of several degenerative disorders, including Duchenne muscular dystrophy.
Studies directed at establishing a causal link between muscular dystrophy and muscle inflammation have revealed a complex dysregulation of the immune response to muscle damage.
During muscular dystrophy, chronic activation of innate immunity causes scarring of skeletal muscle, or fibrosis, which compromises motor function. How immunity is linked to the molecular and cellular regulation of muscle fibrosis was not well defined, until now.
“In our study we found the interaction between two types of cells—a novel stromal progenitor, which is similar to a stem cell, and group 2 innate lymphoid cells (ILC2), which are a type of immune cell that reside in skeletal muscle—promotes the invasion of white blood cells in muscle,” says lead author Jenna Kastenschmidt, an assistant specialist in the University of California, Irvine School of Medicine physiology and biophysics department. “This condition is associated with the elevation of genes that promote muscle tissue scarring found in DMD.”
The new study not only reveals the interaction of cells contributing to DMD, but it illuminates how muscle eosinophilia is regulated. Eosinophils are white blood cells that infiltrate dystrophic muscle causing fibrosis.
In this study in Cell Reports, researchers found elevated levels of eosinophils in DMD muscle compared to control patients. In addition, researchers found the deletion of ILC2s in dystrophic mice mitigated muscle eosinophilia, reducing the expression of genes associated with muscle fibrosis.
These findings contribute to the understanding of the complex regulation of muscle inflammation and fibrosis during muscular dystrophy.
“By further defining the interaction between skeletal muscle-resident immune and stromal cells, we can better understand how chronic inflammation promotes muscle fibrosis and, more importantly, we can facilitate development of novel therapies for DMD,” says senior author Armando Villalta, assistant professor in physiology and biophysics department.
Ongoing work from Villalta’s lab continues to focus on how distinct facets of the immune system regulate DMD pathogenesis and how these processes influence the efficacy and long-term stability of gene replacement therapy.
Source: UC Irvine