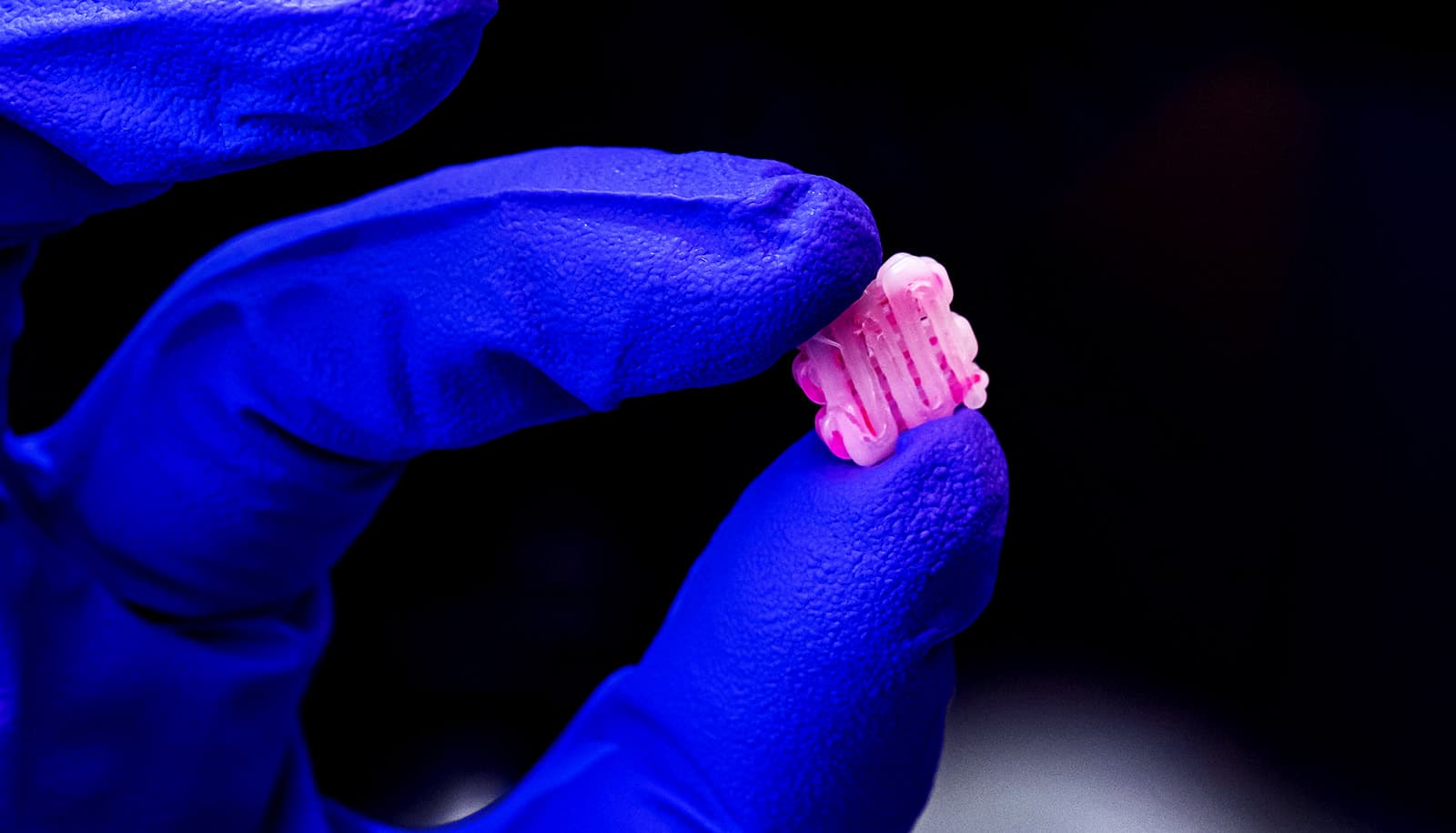
Bioengineers are using grooves to seed sophisticated, 3D-printed tissue-engineering scaffolds with living cells to help heal injuries.
The researchers are literally carving grooves into plastic threads used to build the scaffolds. The grooves are then seeded with cells or other bioactive agents that encourage the growth of new tissue.
The strategy protects cells from the heat and shear stresses that would likely kill them in other scaffold fabrication processes. It also provides a way to layer cells that ultimately become different kinds of tissue, like bone and cartilage, in a mechanically stable platform.
The 3D printer cuts the grooves into a thermoplastic, inserts the cells at the proper temperature, and creates a 3D implant, based on medical images, in a single process.
The research appears in the journal Bioprinting.
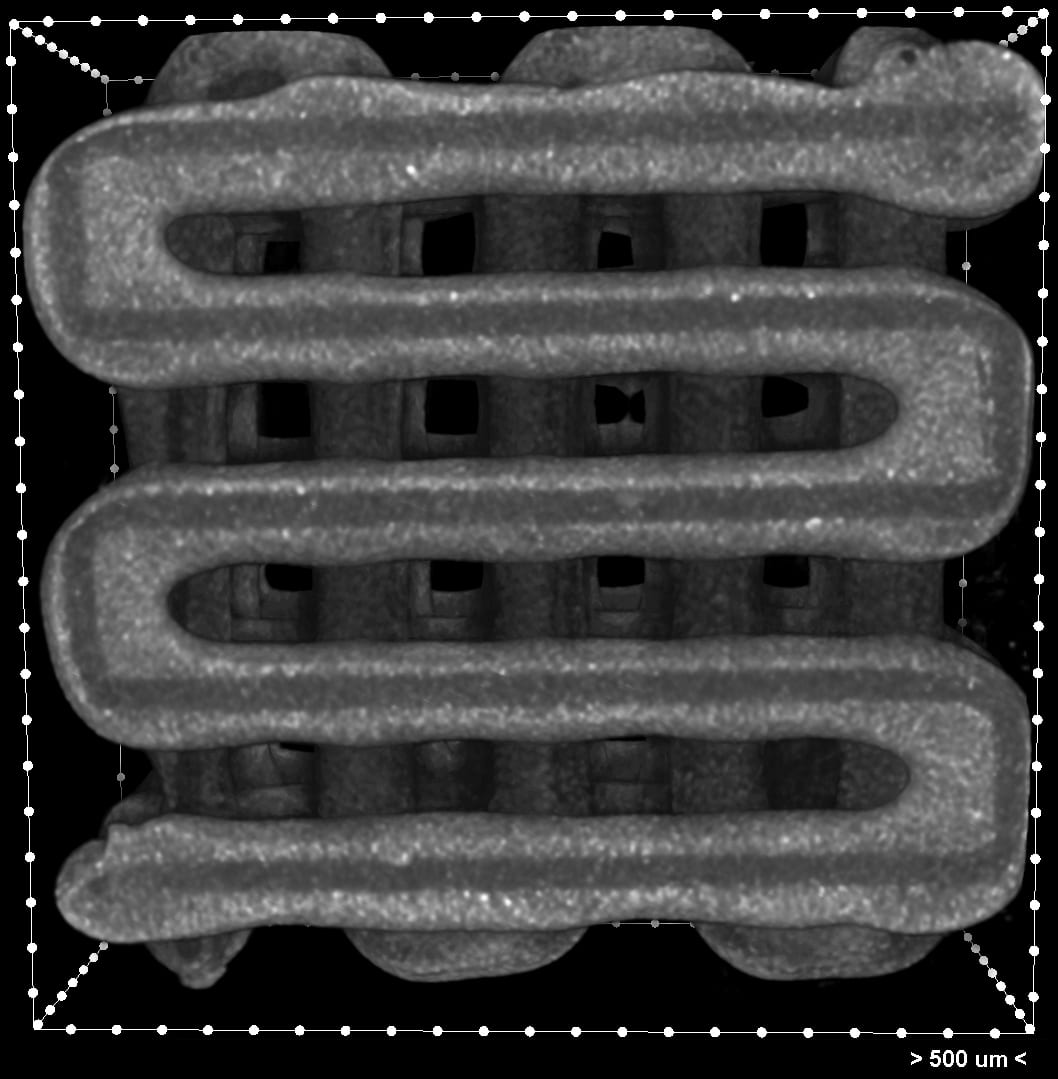
Unlike cell-supporting hydrogel scaffolds under development, this process creates hard implants that would be surgically inserted to heal bone, cartilage, or muscle, says study leader Antonios Mikos of Rice University. Like hydrogels, the biocompatible implants would degrade over time and leave only natural tissue.
“The major innovation here is our ability to spatially load a scaffold that is 3D printed with different cell populations and with different bioactive molecules,” says Mikos, professor of bioengineering, of chemical and biomolecular engineering, of materials science and nanoengineering, and of chemistry.
Until now, 3D-printed scaffolds were generally seeded with uniform distributions of cells, he says. “If we wanted different cell populations at different points in the scaffold, we could not do that. Now we can.”
“The fibers are cylinders that we engrave with a needle to give it a groove as it’s printing,” says Rice research scientist Maryam Elizondo, co-lead author of the paper with alumnus Luis Diaz-Gomez. Once the groove is set and cooled just enough, the printer then deposits a cell-infused ink. “We do that for every fiber for every layer of the scaffold.”
Elizondo compared the grooved threads, which are about 800 microns wide, to taco shells that keep the contents inside without spilling; here, the addition of grooves and ultraviolet-activated crosslinkers keep the cell ink inside. She says it takes about half an hour to completely print a thumbnail-sized implant.
Mikos says the scaffold isn’t limited to cells. “We can also load different growth factors on different levels,” he says. “Very high temperatures would deactivate them, but here we can deposit growth factor-loaded microparticles inside the grooves as they cool. That would preserve the bioactivity of the molecule.”
Coauthors of the paper are from Rice and the University of Texas at Austin. The National Institutes of Health supported the research.
Source: Rice University