A new 3D structure for growing cancer cell cultures could allow doctors to test medications on model tumors they grow from a patient’s own cells, researchers report.
Unlike previous devices, researchers made the new structure from protein fibers that cells know how to modify.
“We can potentially use the cultures to do things like drug testing or single cell analysis, which may help us identify the best treatments for a patient’s cancer,” says Gary Luker, professor of radiology at the University of Michigan.
At present, some patients have samples of their cancer cells grown in mice for drug testing and analysis, but the cancer cells don’t always grow and the process takes months, Luker says.
The 3D scaffold, an advanced petri dish, could allow doctors to get answers about the effectiveness of drugs in days or weeks. Earlier scaffolds, trying to mimic the structure and composition of the gel-like network that binds a collection of cells into a tissue have mixed records.
“Rather than trying to guess at what the tumor cells’ microenvironment ought to be, we’ve made a space where they can create their own cell niche, as they do in the body,” says Stacy Jordahl, a recent chemical engineering PhD graduate and first author on the paper in Advanced Materials.
Natural environment for cell growth
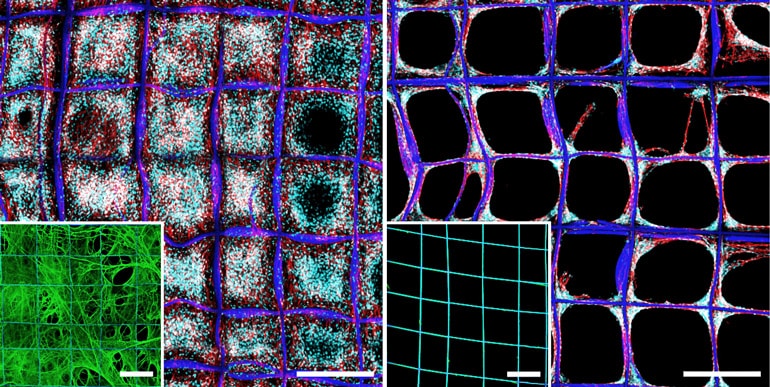
The team created a network of fibronectin, a protein that attaches cells to the connective gel. Cells in tissues stretch out the fibronectin, using it a bit like the two-by-fours of a house frame. However, fibronectin tends to coil up if it’s not held open.
While researchers have used layers of balled-up fibronectin to help cells attach to previous scaffolds, they haven’t taken full advantage of the protein.
Previously, it was painstaking to stretch out fibronectin strands—pulling on them with pipettes, for example—but the new method produces a coating of stretched-out fibronectin without the hard work.
The engineers built a grid of microscale cubicles, each half a millimeter to a side. Then they repeatedly poured a solution containing fibronectin over that surface using a tube that slowly flipped end over end. The tug of the moving liquid was enough to draw the fibronectin out into networks of fibers that interlaced across the whole structure.
“With this engineered way to draw proteins into a network of fibers, we can produce a more natural environment for growing cancer cell cultures that enable us to test drugs or understand cancer biology,” says Joerg Lahann, professor of chemical engineering and director of the Biointerfaces Institute.
Growing cancer cells in culture
Lahann’s team turned the structures over to Luker and Max Wicha, professor of oncology. They used the structures to culture cells removed from breast cancer patients by draining fluid pockets that can accumulate in the abdomen and chest as the disease progresses.
Although cancer cells represent only about 5% of the cells in these fluids, they dominated the cell population after a few days to a week on the fibronectin network. That impressed the cancer researchers.
“There have been a lot of technologies and approaches devised to try to grow cancer cells in culture that haven’t worked so well. Most cancer cells die out when cultured in artificial conditions,” Luker says. “In this system, we could pretty consistently grow out the cultures at least for short periods of time.”
In addition, the cells seemed to change as they grew on the fibronectin network, becoming more like the kind of cells scientists believe spread cancer to other parts of the body. This could offer an advantage when testing cancer drugs, as those cells are most imperative to kill.
However, this bias would hinder experiments exploring cancer biology—for instance, identifying the influences that lead cells to become more aggressive or benign. In the future, the team may investigate whether changes to structure of the fibronectin network can remove this bias.
The National Cancer Institute, National Science Foundation, Department of Defense, and National Institutes of Health funded the work.
Source: University of Michigan



