A new method that uses smartphone-derived images can identify potentially harmful bacteria on the skin and in the mouth, research shows.
The approach can visually identify microbes on skin contributing to acne and slow wound healing, as well as bacteria in the oral cavity that can cause gingivitis and dental plaques.
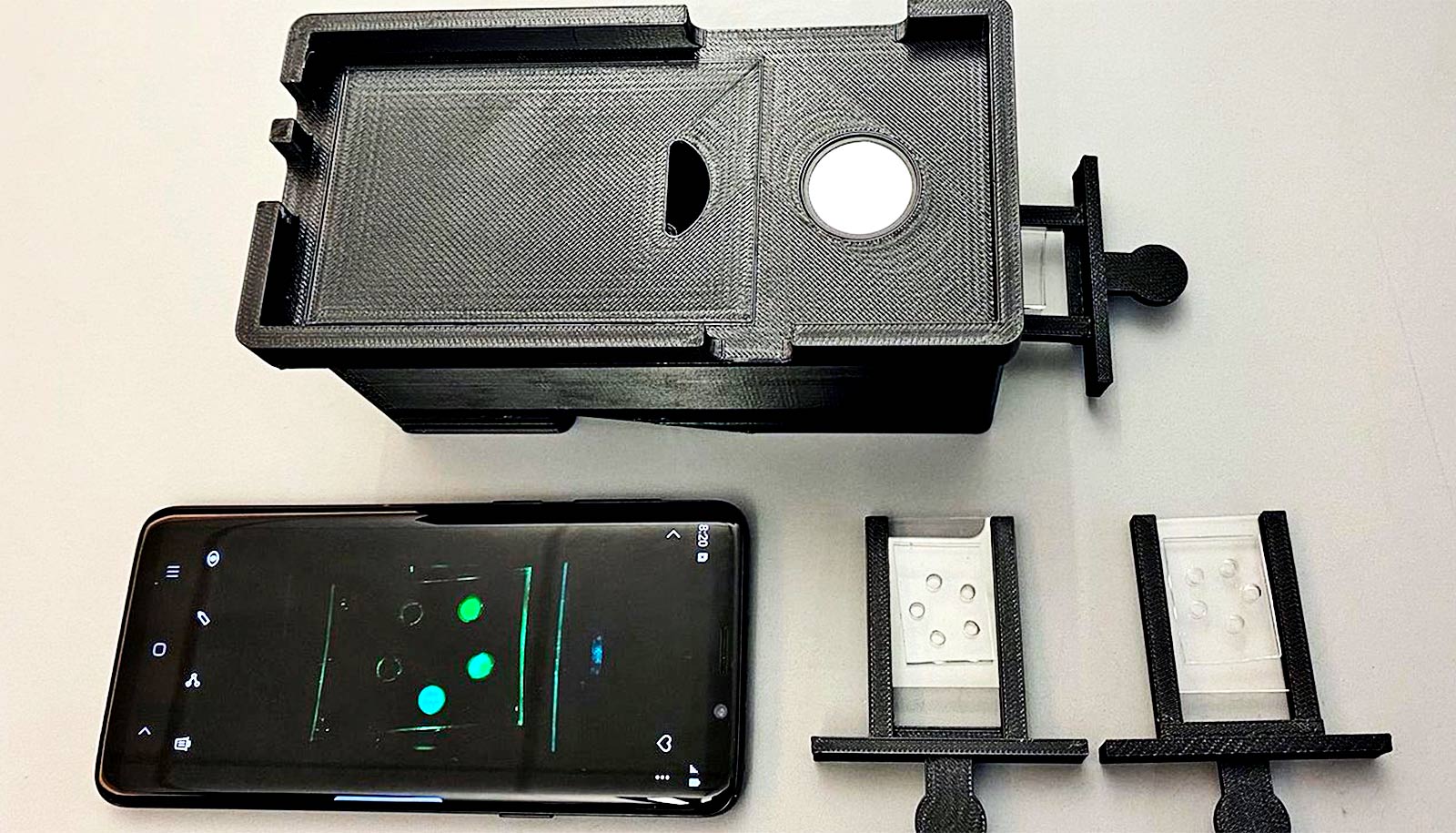
Researchers combined a smartphone-case modification with image-processing methods to illuminate bacteria on images taken by a conventional smartphone camera. This approach yielded a relatively low-cost and quick method that could be used at home.

“Bacteria on skin and in our mouths can have wide impacts on our health—from causing tooth to decay to slowing down wound healing,” says Ruikang Wang, a professor of bioengineering and of ophthalmology at the University of Washington. “Since smartphones are so widely used, we wanted to develop a cost-effective, easy tool that people could use to learn about bacteria on skin and in the oral cavity.”
Bacteria aren’t easy to see using conventional smartphone images. Smartphone cameras are “RGB cameras,” says Wang. They essentially funnel all the different wavelengths of light in the visual spectrum into three different colors—red, green, and blue. Every pixel in a smartphone-generated image is a combination of those colors. But bacteria emit many colors beyond red, green, and blue, which a typical smartphone camera misses.
Wang’s team augmented the smartphone camera’s capabilities by attaching a small 3D-printed ring containing 10 LED black lights around a smartphone case’s camera opening. The researchers used the LED-augmented smartphone to take images of the oral cavity and skin on the face of two research subjects.

“The LED lights ‘excite’ a class of bacteria-derived molecules called porphyrins, causing them to emit a red fluorescent signal that the smartphone camera can then pick up,” says Qinghua He, a doctoral student in bioengineering and lead author of the study, published in Optics and Lasers in Engineering.
Other components in the image—such as proteins or oily molecules our bodies produce, as well as skin, teeth, and gums—won’t glow red under LED. They’ll fluoresce in other colors, He adds.
Many bacteria produce porphyrins as a byproduct of their growth and metabolism. The porphyrins can accumulate on skin and in our mouths where bacteria are present in high amounts, according to coauthor Yuandong Li, a postdoctoral researcher in bioengineering.
“Generally, the more porphyrins you see on skin surface, for example, the greater difficulty you see with wound-healing and acne,” Li says.
The LED illumination gave the team enough visual information to computationally “convert” the RGB colors from the smartphone-derived images into other wavelengths in the visual spectrum. This generates a “pseudo-multispectral” image consisting of 15 different sections of the visual spectrum—rather than the three in the original RGB image. Obtaining this visual information up front would have required expensive and cumbersome lights, rather than using the relatively inexpensive LED black lights, Wang says.
With their greater degree of visual discrimination, the pseudo-multispectral images clearly resolved porphyrin clusters on the skin and within the oral cavity. In addition, though they tailored this method to show porphyrin, researchers could modify the image-analysis pipeline to detect other bacterial signatures that also fluoresce under LED.
“That is the beauty of this technique: We can look at different components simultaneously,” says Wang. “If you have bacteria producing a different byproduct that you want to detect, you can use the same image to look for it—something you can’t do today with conventional imaging systems.”
This initial study’s success could form the basis of new home-based methods to assess basic skin and oral health, the researchers say—providing users with information about whether they need to see a dentist, for example, or consult a doctor about certain types of skin conditions.
The visual system and image-analysis pipeline may also help identify potentially problematic bacteria in other medical contexts, such as wound healing on other parts of the body.
“There are a lot of directions we can go here,” says Wang. “Our bodies are complex environments, and this approach has great potential to look at many types of problems.”
The Washington Research Foundation funded the work.
Source: University of Washington