A robotic swimmer could help researchers better understand the movements of bacteria and other microorganisms.
Just by moving around, microorganisms like bacteria and sperm are performing a remarkable feat. The effects of viscosity are amplified at small scales, which means a microorganism swimming in water is a bit like a person trying to do the backstroke in a tar pit. Scientists still don’t have a complete picture of how they do it.
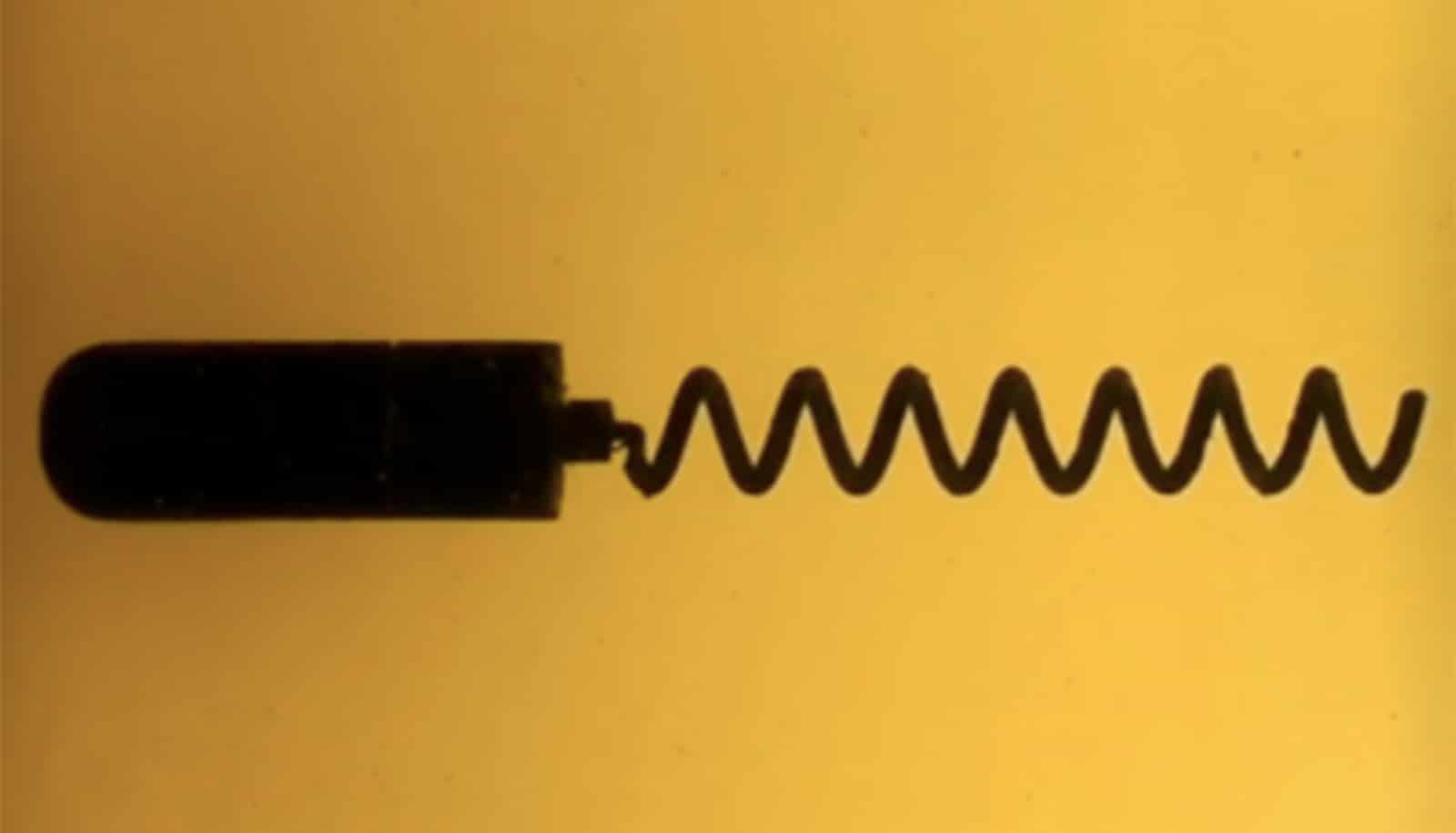
The new tool mimics the action of a flagellum, the corkscrew-like appendage that many microorganisms use to get around. By bringing that swimming action into the macroscopic world, the device makes it easier to study the fluid dynamics of flagellar motion. And because it’s self-propelled, reconfigurable, and remotely controlled, researchers can set up experiments that would be impossible with actual microorganisms.
The researchers hope that the insights produced by the device could be useful in everything from fertility treatment to understanding how infections spread through the body.
“Microorganisms use an incredibly complex form of locomotion,” says Roberto Zenit, a professor in Brown University’s School of Engineering and a senior author of the research in the Review of Scientific Instruments.
“We have mathematical models that make approximations of how it works, but to improve those approximations we need to make detailed measurements of the velocity fields around these organisms. By making a device that can mimic that swimming as closely as possible, we hope to make some of those measurements.”
Zenit had been working for several years on models of microorganism swimming behavior. Previously, he had developed a pill-sized device containing a magnet, which could be made to “swim” using an oscillating magnetic field. The device was a reasonable approximation of bacterial swimming, but Zenit wanted to improve upon it.
“Real bacteria don’t need a magnetic field because they have internal power,” Zenit says. “We wanted to see if we could come up with something that was self-propelled.”
So Zenit turned to Daniel Harris, an assistant professor of engineering whose lab specializes in building custom devices for fluid dynamics research. Zenit and Harris thought that developing a robot prototype might be a good project for a group of Harris’s students.
Under Harris’s direction, a team of undergraduates worked for a semester to come up with a robotic swimmer prototype. One member of the group, Matthew Styslinger, continued to work on the project as a senior capstone project before graduating in 2021. From there, PhD student Asimanshu Das took up the project, adding features and completing the design.
The device is based on the geometry of an E. coli bacterium. It has a cylindrical head, made on a 3D printer, that’s about 6 centimeters (2.36 inches) long and 2 centimeters (0.787 inches) in diameter. The watertight head houses a small motor, a power supply, and other electronics. The motor drives a helical tail, also made on a 3D printer, which is about 9 centimeters (3.54 inches) long. Tails can be swapped in and out to experiment with different helix angles and geometries. A remote control adjusts motor speed and rotation direction.
The team performed a series of benchmark experiments with the device swimming in a mixture of corn syrup and water, which approximates the viscosity of a microscale swimmer plowing through water alone. The results showed that the device’s swimming performance is in line with the predictions of a simple resistive force model, the same theory frequently applied to rationalize the motion of the device’s microscopic counterpart.
Having validated the device, the team now plans a variety of experiments to shed new light on helical swimming.
“What this gives us is the ability to do macroscopic experiments that we have full control over,” Harris says. “Imagine trying to tell a bacterium to swim in a particular direction or change its helix angle. That’s pretty hard to do. But it’s something we can do with this.”
Moving forward, the team plans to make detailed measurements of the flow fields around their swimmers. They hope to shed light on some key questions that remain unanswered, like what happens to flows when a microorganism encounters a hard wall, or how the flow changes when several organisms swim together.
Source: Brown University