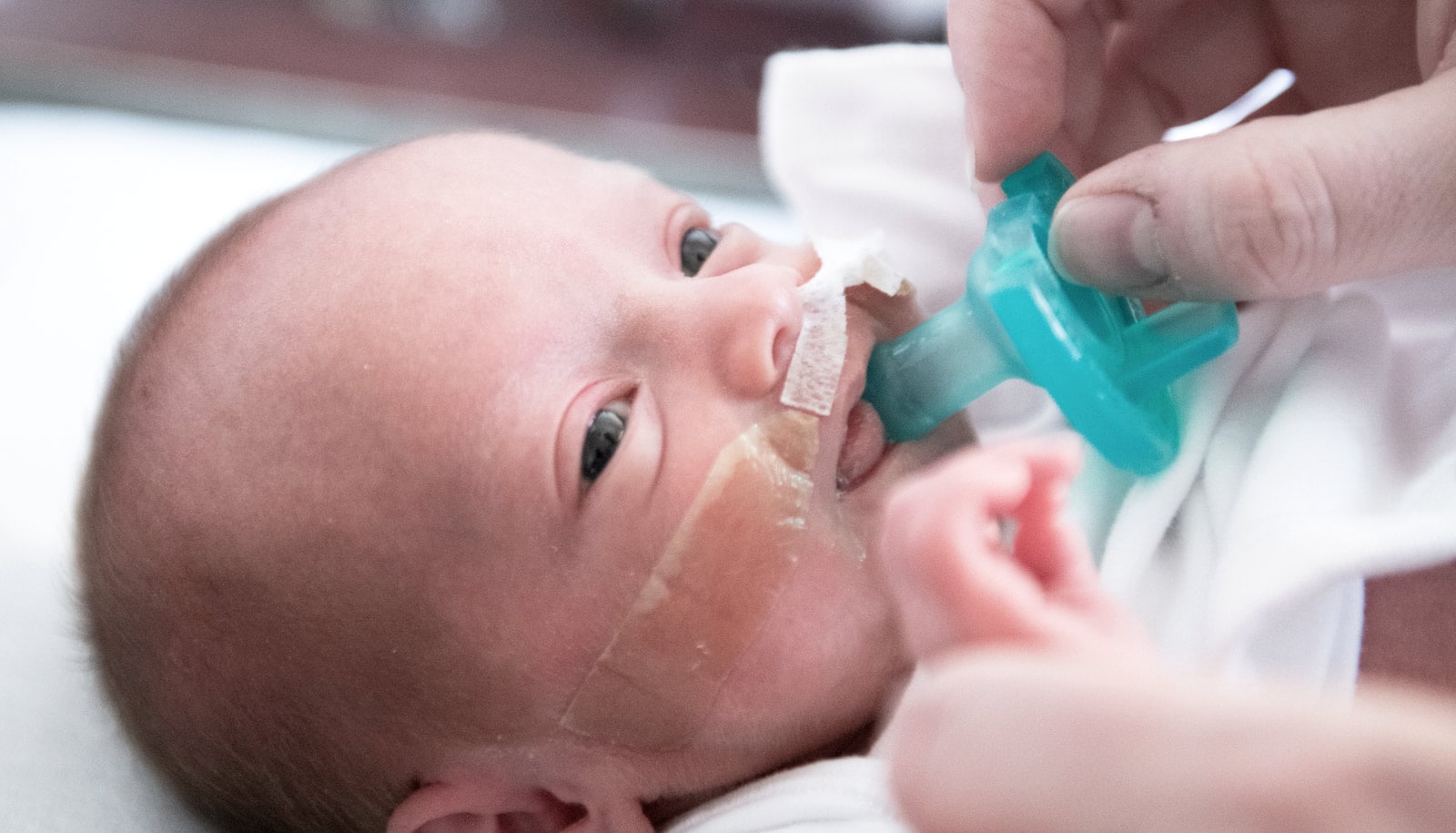
An inhaled drug may offer a way to reduce the risk of life-threatening lung infections like pneumonia, common among premature babies, a study with mice shows.
The underdeveloped lungs and immune systems of premature babies put them at high risk of potentially deadly pneumonia, researchers say, but the drug—an inhaled form of a normal immune protein called GM-CSF—may promote immunity and reduce the risk.
The National Institutes of Health, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Hope Center Transgenic Vectors Core, and the Speed Congenics Facility of the Rheumatic Diseases Core Center funded the work, which appears in Science Advances.
Here, senior author S. Celeste Morley, an associate professor of pediatrics, and of pathology and immunology at Washington University in St. Louis, discusses the findings and explains why she thinks the drug may protect premature lungs now, when previous attempts did not:
Why did you start exploring whether GM-CSF could prevent pneumonia?
Immune cells called macrophages are critical for controlling lung infections, and they appear in the lungs in the first day or two after birth. We know that newborn mice naturally experience a burst of GM-CSF in their lungs in the first 24 to 48 hours after birth that triggers the development of specialized lung macrophages.
We don’t know for sure whether the same thing happens in babies. But we think that full-term babies also get a burst of GM-CSF, and preemies might not. If so, the macrophages in the lungs of premature infants would not develop normally, which could explain why preemies are at such high risk of pneumonia.
What happened when you gave mice GM-CSF?
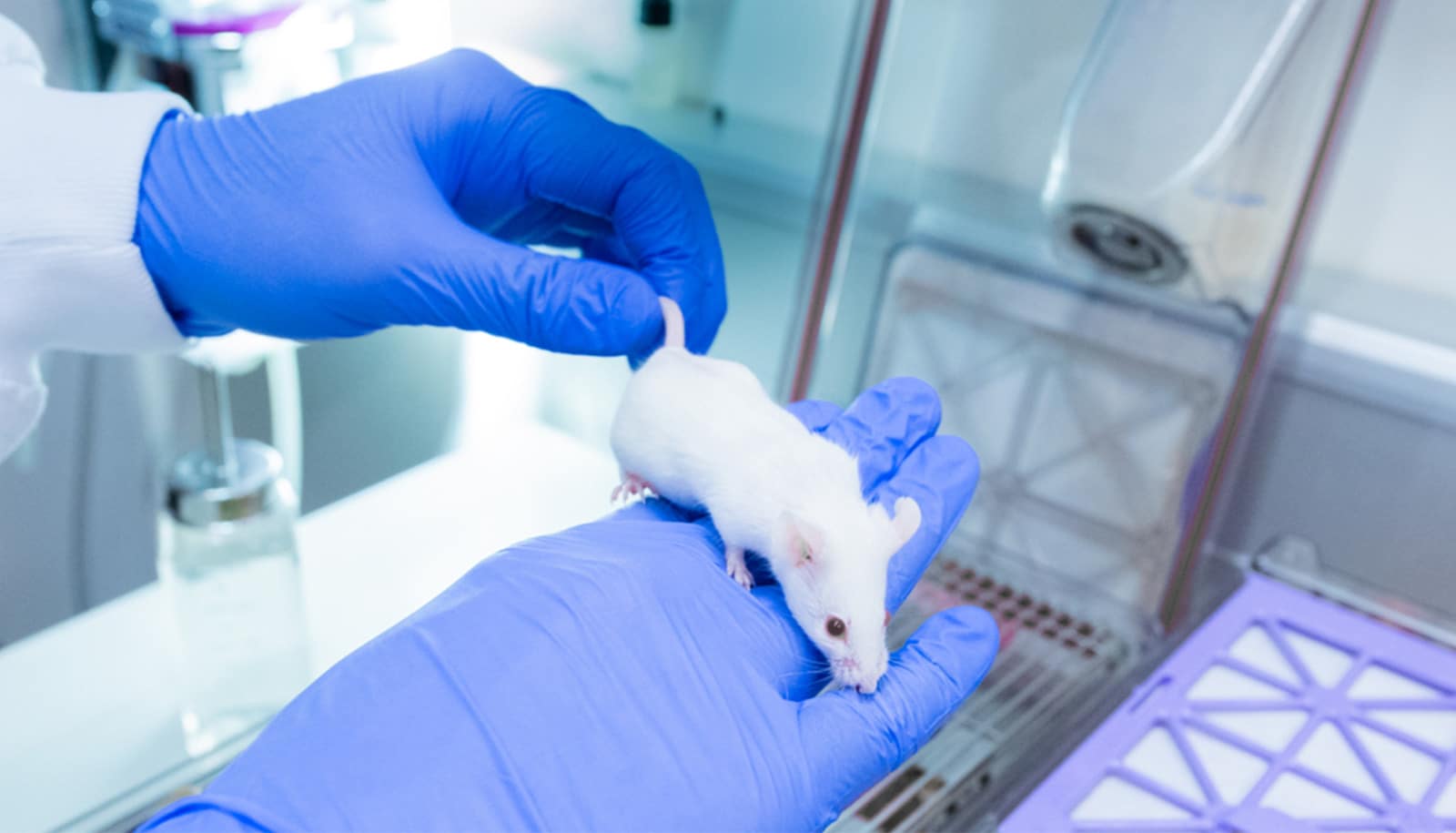
We used mice with a genetic defect that prevents their lung macrophages from maturing normally. We couldn’t study premature mice—they’re just too small to work with. But full-term newborn mice with this genetic defect are very susceptible to bacterial infection and pneumonia, like human preemies, so we used them instead.
We gave these newborn mice GM-CSF intranasally for the first 24 hours after birth. The number of lung macrophages increased immediately, and since they are very long-lived cells, the mice were protected from pneumonia even into adulthood.
GM-CSF has been tried before as a way to protect preemies from pneumonia. Why didn’t it work then, and why do you think it will work now?
People have tried giving premature infants GM-CSF intravenously or subcutaneously. When you give GM-CSF this way, it mobilizes neutrophils, a different immune cell that is also very important for fighting off infections in general, but is not the crucial immune cell type in the lungs.
So it wasn’t a bad idea, but the clinical trials have been disappointing. GM-CSF injections did not lower the risk of pneumonia. What we did differently is we administered the drug to the lungs directly, by inhalation, which triggered the development of lung macrophages instead of neutrophils. And then we saw long-lasting protection against pneumonia.
Could inhaled GM-CSF help protect premature infants from pneumonia?
I hope so. The key point is that it has to be given within the first day after birth. These specialized macrophages are only made in the first couple of days after birth.
After that, the maturation window is over and you’ve missed your chance to intervene. And you have to give it through inhalation so it gets directly to the lungs.
GM-CSF is an FDA-approved drug, and it has been used safely in previous clinical trials in premature infants. We still have some questions to answer, but I hope we can take this into clinical trials soon.