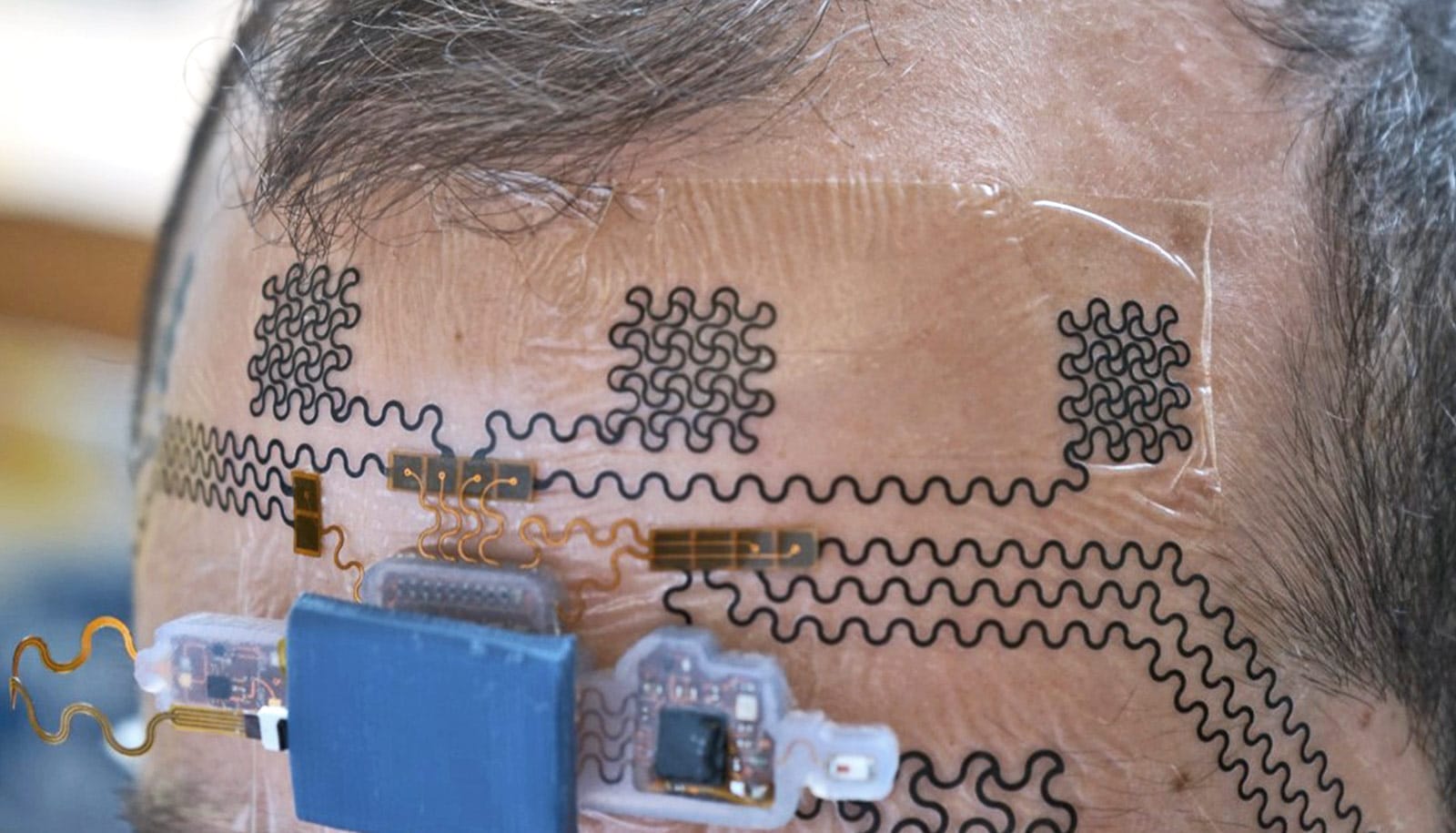
A new device uses fast-moving fluids to insert flexible, conductive carbon nanotube fibers into the brain, where they can help record the actions of neurons.
The microfluidics-based technique promises to improve therapies that rely on electrodes to sense neuronal signals and trigger actions in patients with epilepsy and other conditions.
Eventually, the researchers say, the nanotube-based electrodes could help scientists discover the mechanisms behind cognitive processes and create direct interfaces to the brain that will allow patients to see, to hear, or to control artificial limbs.
The device uses the force applied by fast-moving fluids that gently move insulated flexible fibers into brain tissue without buckling. The new delivery method could replace hard shuttles or stiff, biodegradable sheaths now used to deliver wires into the brain. Both can damage sensitive tissue along the way.
Lab and in vivo experiments showed how the microfluidic devices force a viscous fluid to flow around a thin fiber electrode. The fast-moving fluid slowly pulls the fiber forward through a small aperture that leads to the tissue. Once it crosses into the tissue, tests showed that the wire, though highly flexible, stays straight.
“The electrode is like a cooked noodle that you’re trying to put into a bowl of Jell-O,” says project leader Jacob Robinson, an engineer at Rice University. “By itself, it doesn’t work. But if you put that noodle under running water, the water pulls the noodle straight.”
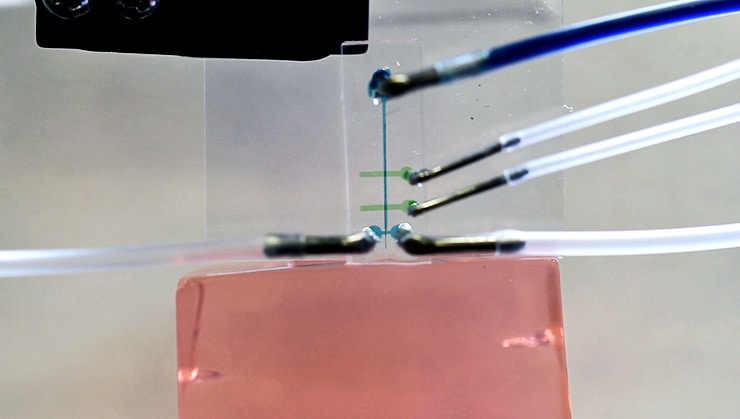
The wire moves slowly relative to the speed of the fluid. “The important thing is we’re not pushing on the end of the wire or at an individual location,” says coauthor Caleb Kemere, an electrical and computer engineer who specializes in neuroscience. “We’re pulling along the whole cross-section of the electrode and the force is completely distributed.”
“It’s easier to pull things that are flexible than it is to push them,” Robinson says.
“That’s why trains are pulled, not pushed,” adds chemist and coauthor Matteo Pasquali. “That’s why you want to put the cart behind the horse.”
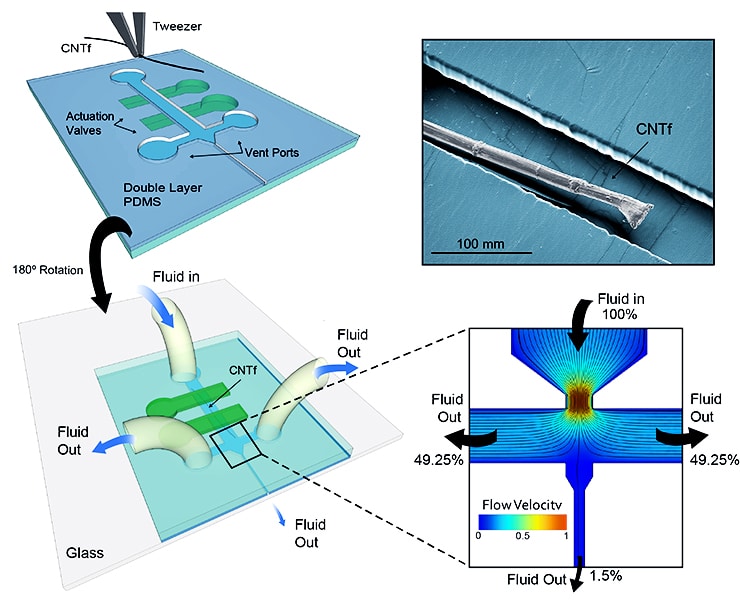
The fiber moves through an aperture about three times its size but still small enough to let very little of the fluid through. Robinson says none of the fluid follows the wire into brain tissue (or, in experiments, the agarose gel that served as a brain stand-in), Robinson says.
There’s a small gap between the device and the tissue, Robinson says. The small length of fiber in the gap stays on course like a whisker that remains stiff before it grows into a strand of hair.
“We use this very short, unsupported length to allow us to penetrate into the brain and use the fluid flow on the back end to keep the electrode stiff as we move it down into the tissue,” he says.
Amputees could control robotic arm with brain implant
“Once the wire is in the tissue, it’s in an elastic matrix, supported all around by the gel material,” Pasquali says. “It’s supported laterally, so the wire can’t easily buckle.”
Carbon nanotube fibers conduct electrons in every direction, but to communicate with neurons, they can be conductive at the tip only, Kemere says. “We take insulation for granted. But coating a nanotube thread with something that will maintain its integrity and block ions from coming in along the side is nontrivial,” he says.
Sushma Sri Pamulapati, a graduate student in Pasquali’s lab, developed a method to coat a carbon nanotube fiber and still keep it between 15 to 30 microns wide, well below the width of a human hair.
“Once we knew the size of the fiber, we fabricated the device to match it,” Robinson says. “It turned out we could make the exit channel two or three times the diameter of the electrode without having a lot of fluid come through.”
The technology may eventually be scaled to deliver multiple microelectrodes that are closely packed into the brain all at once, making it safer and easier to embed implants.
“Because we’re creating less damage during the implantation process, we might be able to put more electrodes into a particular region than with other approaches,” Robinson says.
Soft nanotube fibers may improve brain electrodes
The researchers describe their work in the journal Nano Letters.
Flavia Vitale, a Rice alumna and now a research instructor at the University of Pennsylvania, and Daniel Vercosa, a Rice graduate student, are lead authors of the paper. Other coauthors are from Rice; the University of Texas Health Science Center at Houston; and the University of Parma, Italy.
The Defense Advanced Research Projects Agency, the Welch Foundation, the National Science Foundation, the Air Force Office of Scientific Research, the American Heart Association, the National Institutes of Health, the Citizens United for Research in Epilepsy Taking Flight Award, and the Dan L. Duncan Foundation supported the work.
Source: Rice University