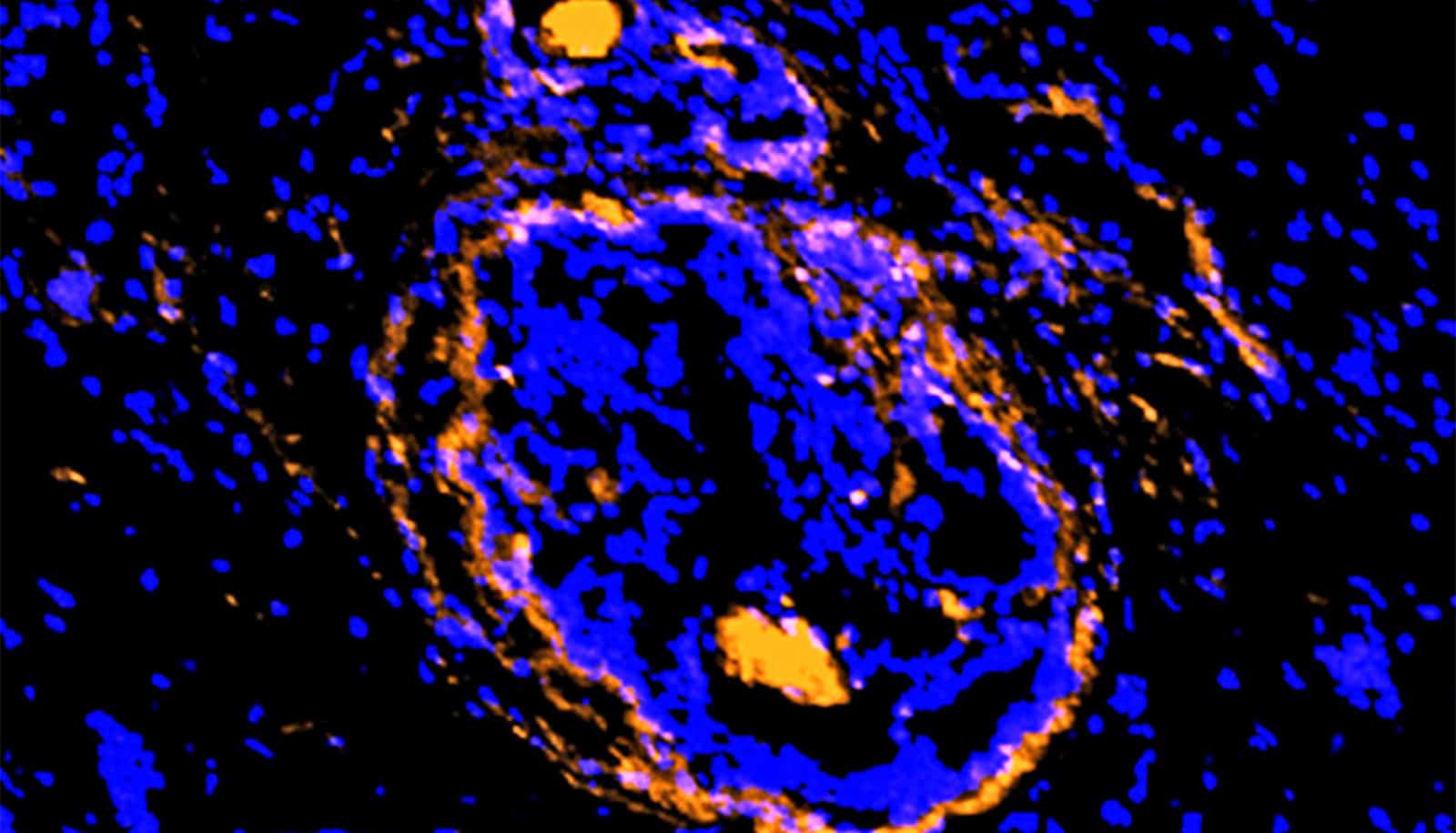
If clinicians could stop mutations of the KRAS gene in pancreatic cancer, the chances of improving treatment for this deadly form of cancer would increase.
These mutations occur in more than 90% of pancreatic cancer cases and drastically reduce response to immunotherapy.
A new study in Nature Communications takes an initial step toward better understanding how KRAS drives immune evasion and demonstrates a lowering of the KRAS activity resulting in a more favorable immune environment to fight cancer.
Previous strategies to block the KRAS oncogene therapeutically have focused on counteracting its growth-promoting role in cancer.
“Instead, our study shows that oncogenic KRAS plays a profound immunosuppressive role in cancer maintenance, and that treatment of cancer will be improved by simultaneously inhibiting KRAS and activating immune pathways suppressed by the cancer,” says Oleksi Petrenko, research assistant professor in the department of microbiology and immunology in the Renaissance School of Medicine at Stony Brook University.
The researchers used an animal model of pancreatic ductal adenocarcinoma (PDAC) to demonstrate that at an advanced tumor stage dependence on KRAS for tumor growth is reduced and is manifested in the suppression of anti-tumor immunity. KRAS-deficient cells retain the ability to form tumors in immunodeficient mice. However, they fail to evade the immune system in immunocompetent wild-type mice, triggering a strong anti-tumor response.
“The key insight of the paper’s findings is that mutations in KRAS not only promote tumor growth but also keep tumors ‘cold,’ rather than ‘hot’ when cytoxic T-cells are attacking the cancerous cells,” says coauthor Scott Powers, professor in the pathology department in the Renaissance School of Medicine and director of clinical cancer genomics at the Stony Brook University Cancer Center.
“We believe that this study clearly demonstrates the ability of oncogenic KRAS to corrupt our anti-tumor immune responses,” adds Nancy C. Reich, coauthor and professor in the department of microbiology and immunology. “It highlights the need to develop therapeutic interventions that not only target KRAS pathways but that engage immune cell defense.”
Support for the research came in part from the National Institutes of Health’s National Cancer Institute, the Carol M. Baldwin Breast Cancer Research Award to Reich, and the Catacosinos Cancer Research Award to Petrenko.
Source: Stony Brook University