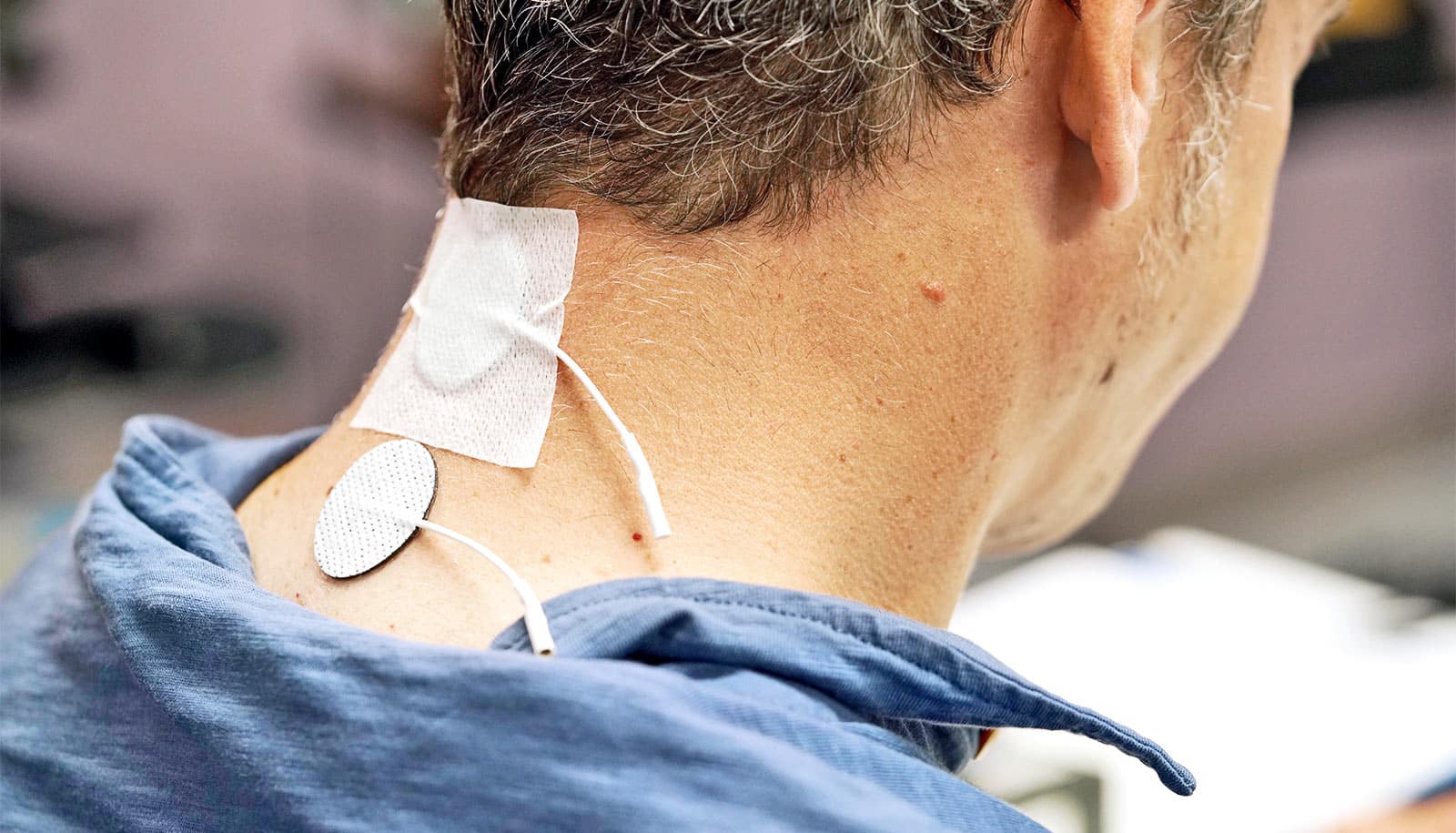
A new treatment that combines physical therapy and a noninvasive method of stimulating nerve cells may offer some people with spinal cord injuries use of their hands and arms.
Almost 18,000 Americans experience traumatic spinal cord injuries every year. Many of these people are unable to use their hands and arms and can’t do everyday tasks such as eating, grooming, or drinking water without help.
The new treatment helped six Seattle area participants regain some hand and arm mobility. That increased mobility lasted at least three to six months after treatment had ended. The findings appear in the journal IEEE Transactions on Neural Systems and Rehabilitation Engineering.

“We use our hands for everything—eating, brushing our teeth, buttoning a shirt. Spinal cord injury patients rate regaining hand function as the absolute first priority for treatment. It is five to six times more important than anything else that they ask for help on,” says lead author Fatma Inanici, a senior postdoctoral researcher in electrical and computer engineering at the University of Washington who completed this research as a doctoral student of rehabilitation medicine in the School of Medicine.
“At the beginning of our study,” Inanici says, “I didn’t expect such an immediate response starting from the very first stimulation session. As a rehabilitation physician, my experience was that there was always a limit to how much people would recover. But now it looks like that’s changing. It’s so rewarding to see these results.”
‘A dramatic change’
After a spinal cord injury, many patients do physical therapy to help them attempt to regain mobility. Recently, a series of studies have shown that implanting a stimulator to deliver electric current to a damaged spinal cord could help paralyzed patients walk again.
For the current study, researchers combined stimulation with standard physical therapy exercises, but the stimulation doesn’t require surgery. Instead, it involves small patches that stick to a participant’s skin like a Band-Aid. These patches are placed around the injured area on the back of the neck where they deliver electrical pulses.

The researchers recruited six people with chronic spinal cord injuries. All participants had been injured for at least a year and a half. Some participants couldn’t wiggle their fingers or thumbs while others had some mobility at the beginning of the study.
To explore the viability of using the skin-surface stimulation method, the researchers designed a five-month training program. For the first month, the researchers monitored participants’ baseline limb movements each week. Then for the second month, the team put participants through intensive physical therapy training, three times a week for two hours at a time. For the third month, participants continued physical therapy training but with stimulation added.
“We turned on the device, but they continued doing the exact same exercises they did the previous month, progressing to slightly more difficult versions if they improved,” Inanici says.
For the last two months of the study, researchers divided the participants into two categories: Participants with less severe injuries received another month of training alone and then a month of training plus stimulation. Patients with more severe injuries received the opposite—training and stimulation first, followed by only training second.
While some participants regained some hand function during training alone, all six saw improvements when stimulation was combined with training.
“Both people who had no hand movement at the beginning of the study started moving their hands again during stimulation, and were able to produce a measurable force between their fingers and thumb,” says senior author Chet Moritz, associate professor of electrical and computer engineering, rehabilitation medicine, and physiology and biophysics. “That’s a dramatic change, to go from being completely paralyzed below the wrists down to moving your hands at will.”
Improvements beyond hands and arms
Some participants also noticed other improvements, including a more normal heart rate and better regulation of body temperature and bladder function.
The team followed up with participants for up to six months after training and found that these improvements remained, despite no more stimulation.
“We think these stimulators bring the nerves that make your muscles contract very close to being active. They don’t actually cause the muscle to move, but they get it ready to move. It’s primed, like the sprinter at the start of a race,” says Moritz, who is also the co-director of the Center for Neurotechnology.
“Then when someone with a spinal cord injury wants to move, the few connections that might have been spared around the injury are enough to cause those muscles to contract.”
The research is moving toward helping people in the clinic. The results of this study have already informed the design of an international multi-site clinical trial that Moritz will co-lead.
“We’re seeing a common theme across universities—stimulating the spinal cord electrically is making people better,” Moritz says. “But it does take motivation. The stimulator helps you do the exercises, and the exercises help you get stronger, but the improvements are incremental. Over time, however, they add up into something that’s really astounding.”
The Center for Neurotechnology, the Washington State Spinal Cord Injury Consortium, and the Christopher and the Dana Reeve Foundation funded the work.
Source: University of Washington