A new technology could accurately detect and classify cancer cells, and determine the aggressiveness of the disease from the least invasive biopsies, researchers report.
With the technology, called STAMP (Sequence-Topology Assembly for Multiplexed Profiling), it’s possible to get comprehensive disease information faster, at a much earlier stage of the clinical workflow, which means doctors can decide on and administer treatments earlier and more effectively.
A biopsy, which involves removing a small amount of tissue from the body, is the main way doctors diagnose most cancers. While doctors prefer less invasive biopsy procedures, they can yield insufficient samples, resulting in incomplete and/or inconclusive diagnosis. Doctors can only make a definitive diagnosis and further assessments (such as cancer staging) after surgery; and they then use that information to guide subsequent treatment decisions.
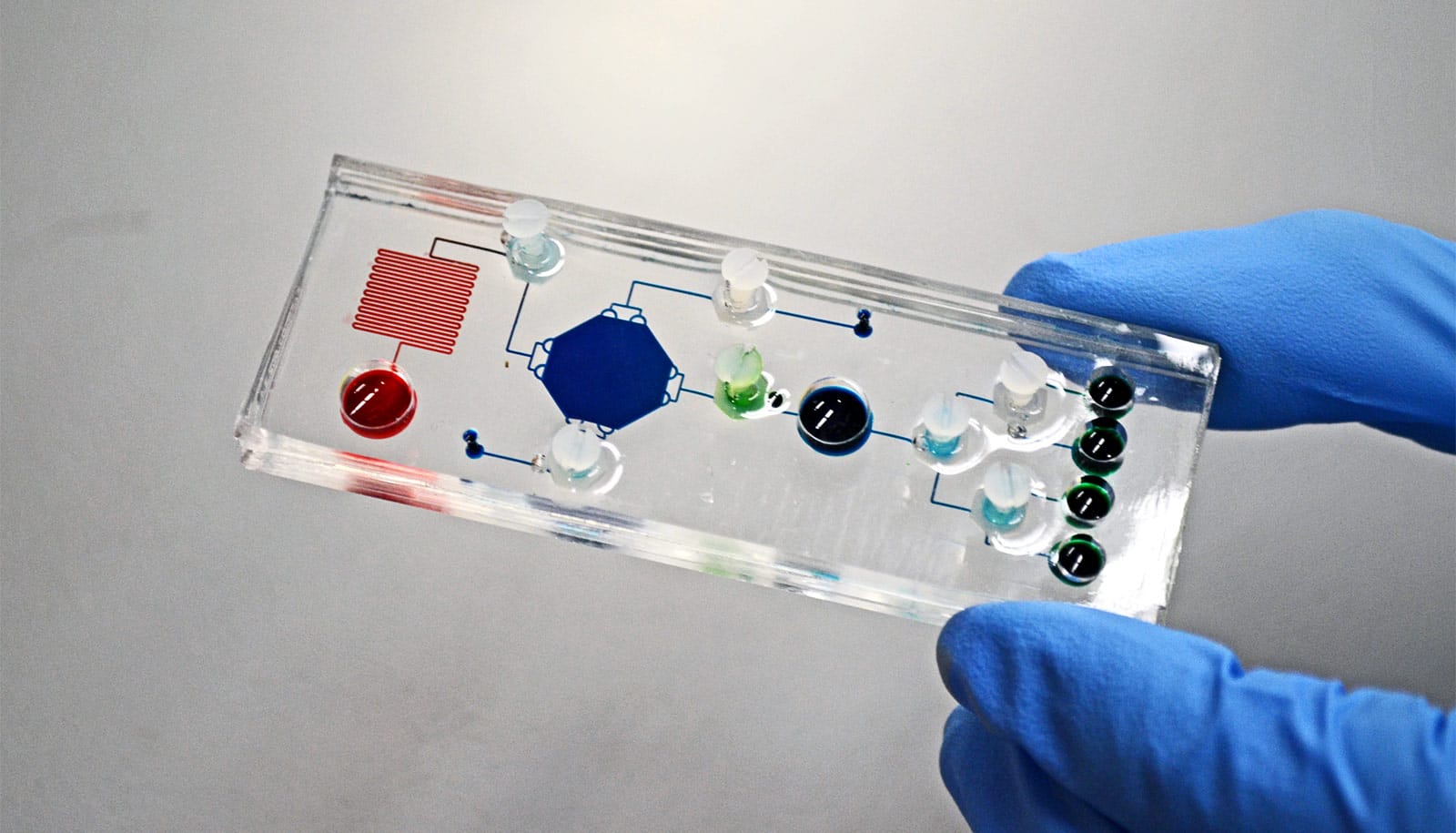
The STAMP technology overcomes many challenges of the clinical workflow to allow early and informative cancer diagnostics. STAMP uses programmable DNA barcodes to measure billions of protein markers in a single test—the amount as well as the distribution of these protein markers in a cell—from a small clinical sample.
Using breast cancer as a model, STAMP can achieve a high diagnostic accuracy of above 94%, comparable with gold-standard tissue pathology. The technology also reveals important clinical information that doctors can currently can only obtain through post-surgery tissue analysis—all directly from a fine needle aspiration (FNA) biopsy, the least invasive form of biopsy.
“Our STAMP technology leverages the unique properties of DNA to form 3D barcodes. These barcodes can be used to measure diverse protein markers as well as detect the markers’ specific locations in cells,” says Shao Huilin, assistant professor at the Institute for Health Innovation & Technology at the National University of Singapore.
“By mapping these marker distribution patterns in cells, STAMP can provide an early indication of disease aggressiveness. Current pathology techniques only measure a small subset of protein markers and require several days of extensive processing. In comparison, STAMP is a million times more sensitive, provides highly informative analysis from scarce samples, and can be completed in as little as two hours.”
Billions of proteins in 1 test
Comprehensive analysis of protein expression and distribution holds great promise for discovery of biomarkers, early disease detection, and more-informed consideration of treatment options. However, current approaches involve imaging and microscopy techniques, which are complex, time-consuming, and have a limited multiplexing capability. The researchers conceptualized and developed STAMP to address these challenges.
DNA exists in nature as long “ribbons” to store massive amounts of genetic information through its combination of base codes. Aside from this well-known linear form, DNA can be precisely engineered to fold into 3D nanostructures with enhanced stability.
STAMP leverages these two important properties of DNA—a large capacity to store information as well as its programmability to fold and unfold into different structures—to engineer convertible barcodes. Researchers can use the STAMP barcodes to measure billions of protein markers in a single test and identify the specific locations of these protein markers in cells.
“To label diverse protein markers in cells, STAMP uses DNA barcodes which are folded as compact nanostructures. These 3D barcodes achieve a high labelling efficiency and remain stable against biological degradation. Each 3D barcode is further given a localization label to encode protein marker location and distribution within the cell,” says first author Noah Sundah, a doctoral student from NUS iHealthtech and NUS Biomedical Engineering.
“To perform analysis, these 3D barcodes are unfolded on-demand through heating to release a pool of linear DNA, which can be easily analyzed using established technologies such as PCR and DNA sequencing. In this way, the expression of a very large number of protein markers and their distribution in cells can be sensitively measured in a single test,” Sundah says.
Fast and cheap
To facilitate clinical processing and measurement, the research team implemented the STAMP technology on a small microfluidic chip that is about half the size of a credit card. Even small amounts of clinical samples could generate results and researches estimate each test to cost about $36 US.
To validate the technology’s performance, researchers conducted a clinical study involving 69 breast cancer patients. They collected FNA biopsies from each patient and analyzed them using STAMP. For comparison, they also performed gold-standard pathology analysis on post-surgery tissues for all patients.
The STAMP analysis of the FNA samples demonstrated a high level of accuracy—more than 94% for cancer diagnosis and subtyping, making it equally accurate as pathology analysis of surgical tissues. Importantly, based on its comprehensive protein marker analysis, STAMP was also able to accurately identify disease aggressiveness from the scarce biopsy samples.
The researchers have filed for a provisional patent for the technology. They expect it to reach the market within the next five years and hope to expand its application to other types of cancer, including brain, lung, and gastric cancer.
The research appears in Nature Biomedical Engineering.
Source: NUS