Scientists report a new method to determine how bacteria sense contact with surfaces, an action that triggers the formation of biofilms—multicellular structures that cause major health issues in people and threaten critical infrastructure, such as water and sewer systems.
It’s estimated that biofilms contribute to about 65 percent of human infections and cause billions in medical costs each year. They infamously played a role in unsafe coliform levels in the water supply of 21 million Americans in the early 1990s and, more recently, likely played a role in several outbreaks of Legionnaire’s disease in Flint, Michigan. They also regularly contribute to global cholera outbreaks.
Biofilms cause serious damage in industry, including clogging water filtration systems or slowing down cargo ships by “biofouling” the vehicles’ hulls, costing an estimated $200 billion per year in the US alone. There are also beneficial biofilms, such as those that aid digestion or help break down organic matter in the environment.
The researchers, led by Indiana University biology professor Yves Brun, discovered the way bacteria detect and cling to surfaces. The researchers also discovered a method to trick bacteria into thinking they are sensing a surface.
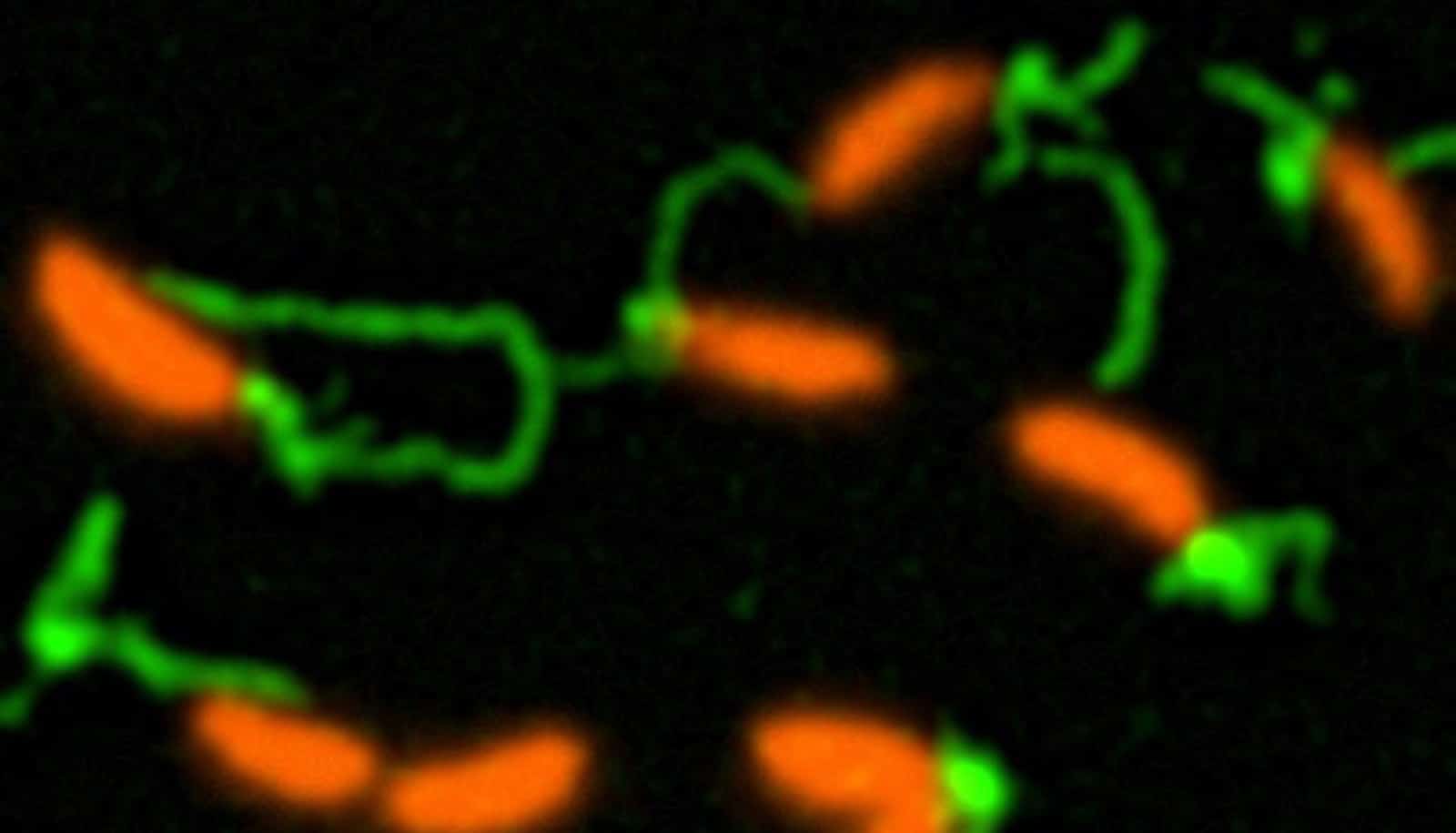
(Credit: Courtney Ellison/Indiana U.)
The team showed that bacteria use ultra-thin hair-like appendages called pili that extend from the cell and retract dynamically to feel and stick to surfaces and ultimately produce biofilms. The pili stop moving after sensing a surface, after which the bacteria start producing an extremely sticky substance, or “bioadhesive,” that drives attachment to surfaces and biofilm formation.
To fool the bacteria into sensing a surface, Brun’s team attached a large maleimide molecule to the pili to effectively block the hair-like structures’ movement.
“It’s like trying to pull a rope with a knot in the middle through a hole—the maleimide molecule can’t pass through the hole the cell uses to extend and retract the pili,” says Courtney Ellison, the study’s lead author and a PhD student in Brun’s lab.
“These results told us the bacteria sense the surface like how a fisherman knows their line is stuck under water,” Brun adds. “It’s only when they reel in the line that they sense a tension, which tells them their line is caught. The bacteria’s pili are their fishing lines.”
Dyed ‘limbs’
The discovery, which appears in the journal Science, is possible due to the team’s new method to observe how bacteria use pili to spread biofilms. They accomplished this observation with expertly delivered fluorescence dyes—delivered on the back of smaller maleimide molecules—that revealed the movement of these microscopic “limbs.”
“By using fluorescent dyes to label these microscopic structures, we’re able to produce images that show the first direct evidence of the role that pili play to detect surfaces,” Brun says.
In order to observe the movement of pili, the team had to overcome a challenge: how to visualize the extremely thin structures and their movement. They did this by substituting a single amino acid within the chain of amino acids that comprise the pili with another amino acid called a cysteine. The maleimide, which delivered the fluorescent dyes to the pili proteins, binds to the cysteine. The maleimide is also the molecule used to deliver the large molecule to the cysteine in the pili protein to physically block the pili movement.
Use biofilm’s own enzymes to defeat its ‘armor’
“It’s like switching on a light in a dark room,” Ellison says. “Pili are composed of thousands of protein subunits called pilins, with each protein in the chain composed of amino acids arranged like a tangled mess of burnt-out Christmas lights. Swapping out a single light can illuminate the whole string.”
More pili
Engineering a cysteine molecule that could replace an amino acid in the pilins without affecting the pili’s overall behavior was a major challenge, she adds. The researchers used Caulobacter crescentus bacteria, which are common to lab experiments.
“We also used this method in this study to visualize the three types of pili produced by Vibrio cholerae, a bacterium that causes cholera,” says study coauthor Ankur Dalia, an assistant professor of biology. “Pili are critical to many aspects of Vibrio’s virulence, and we are now using this powerful tool to understand how they use them.”
Magnetic nanoparticles can bust biofilms
Next, Brun and colleagues hope to unravel precise mechanisms that link pili movement and bioadhesive production, as the two processes appear related but the exact nature of the connection remains unknown.
“The more we understand about the mechanics of pili in biofilm formation and virulence, the more we can manipulate the process to prevent harm to people and property,” Brun says.
Additional coauthors of the study are from Indiana University, Emory University, and City University of New York-Brooklyn College.
Support for the study came from the National Institutes of Health and the National Science Foundation.
Source: Indiana University



