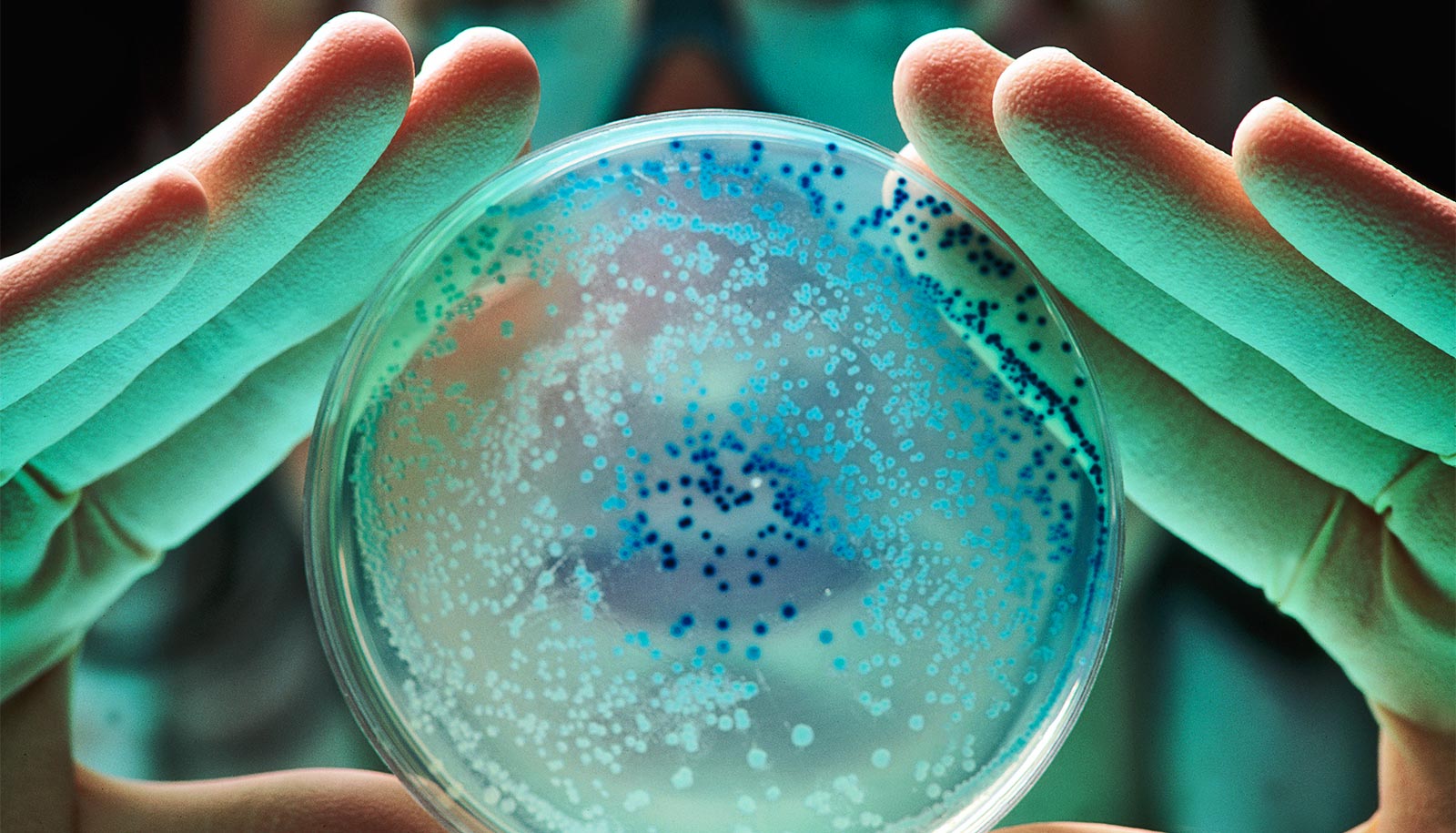
New research illuminates key steps in the process of how bacteria turn antibiotics into food. The finding could lead to new ways to eliminate antibiotics from land and water, the researchers say.
“It’s just carbon, and wherever there’s carbon, somebody will figure out how to eat it.”
“Ten years ago we stumbled onto the fact that bacteria can eat antibiotics, and everyone was shocked by it,” says senior author Gautam Dantas, associate professor of pathology and immunology, of molecular microbiology, and of biomedical engineering at Washington University in St. Louis School of Medicine.
“But now it’s beginning to make sense. It’s just carbon, and wherever there’s carbon, somebody will figure out how to eat it. Now that we understand how these bacteria do it, we can start thinking of ways to use this ability to get rid of antibiotics where they are causing harm.”
Drug resistance is a serious and worsening problem that threatens to set medical care back to a time when scientists had yet to discover antibiotics and infectious disease was the number one cause of death worldwide.
Modern industrial and agricultural practices are speeding up the rise of antibiotic resistance by saturating the environment with active drugs. In India and China, which together produce the vast majority of the world’s antibiotics, pharmaceutical factories sometimes dump antibiotic-laden waste into local waterways. In the United States, some farmers add antibiotics to their animal feed to help their livestock grow, which produces waste loaded with the drugs.
Bacteria easily share genetic material. So when antibiotics infiltrate the water and soil, resident bacteria respond by spreading antibiotic resistance genes through the community.
Researchers wanted to understand how some environmental bacteria not only withstand antibiotics, but feed on them. They studied four distantly related species of soil bacteria that all flourish on a diet of penicillin alone.
Penicillin was the first antibiotic scientists discovered, but it has fallen out of favor because of resistance. Other members of the penicillin family such as amoxicillin and ampicillin are still effective and widely prescribed to treat bacterial infections.
They found three distinct sets of genes that became active while the bacteria ate penicillin but inactive while the bacteria ate sugar. The three sets of genes correspond to three steps bacteria take to transform a lethal compound into a meal.
All of the bacteria start by neutralizing the dangerous part of the antibiotic. Once the toxin is disarmed, they snip off a tasty portion and eat it.
Treating manure doesn’t remove all the antibiotics
Understanding the steps involved in converting an antibiotic into food could help bioengineer bacteria to clean up soil and waterways contaminated with drugs, slowing the spread of drug resistance.
The soil bacteria that naturally eat antibiotics are finicky and difficult to work with. But a more tractable species such as E. coli could potentially be engineered to feed on antibiotics in polluted land or water.
Researchers showed they could give E. coli the ability to survive and thrive on penicillin. The bacterium normally requires sugar, but with some genetic modification and the addition of a key protein, it flourished on a sugar-free diet of penicillin.
“With some smart engineering, we may be able to modify bacteria to break down antibiotics in the environment,” Crofts says.
Any such bioengineering project would have to include a plan to speed up the antibiotic-eating process. The way soil bacteria naturally remove antibiotics from the environment is effective but slow. They couldn’t possibly handle the amounts of antibiotics near pharmaceutical factories and in sewage facilities.
“You couldn’t just douse a field with these soil bacteria today and expect them to clean everything up,” Dantas says. “But now we know how they do it. It is much easier to improve on something that you already have than to try to design a system from scratch.”
Digging in dirt unearths new kind of antibiotics
The National Institutes of Health; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute of General Medical Sciences; the National Institute of Allergy and Infectious Diseases; the National Institute of Child Health and Development; the National Human Genome Research Institute; the Edward Mallinckrodt, Jr. Foundation; the National Science Foundation; and the Mr. and Mrs. Spencer T. Olin Fellowship funded the work.