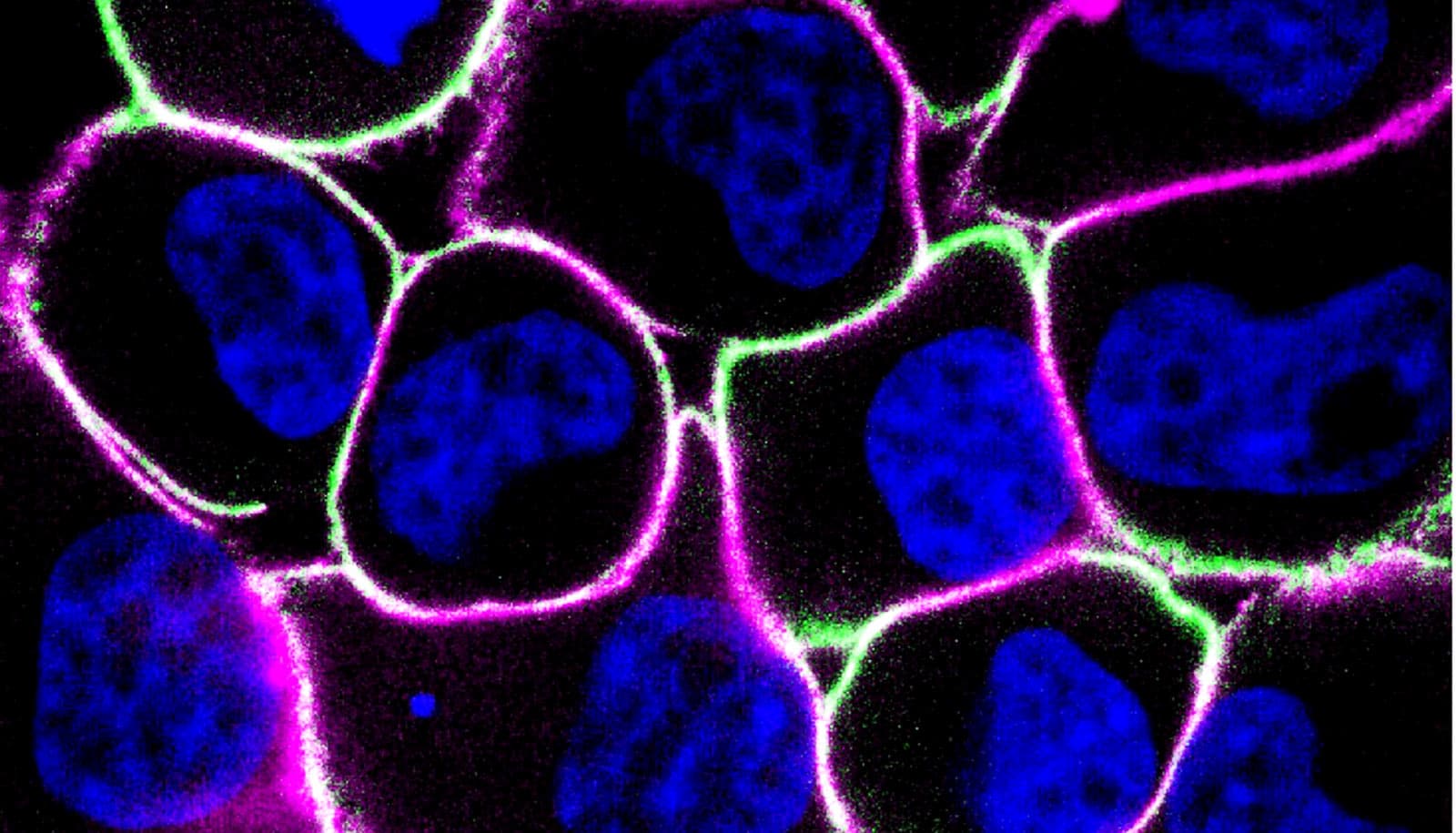
Researchers have created a treatment that could one day unlock a universal strategy for treating some of the hardest-to-treat cancers—like those in the brain, breast, and colon—by teaching the immune system to see what it usually misses.
Their experimental approach worked against those kinds of cancers in lab tests and didn’t damage healthy tissues. Importantly, it also stopped cancer from returning.
While the therapy is still in early stages of development, it builds on well established, safe technologies, giving the treatment a clearer, quicker path to clinical trials and patient care.
Reported in the journal Nature Cancer, their technique is a one-two punch that flags tumor cells so they can be recognized and then eliminated by specially enhanced T cells from the patient’s own immune system.
The team led by Gabe Kwong found they could mark tumors using a synthetic antigen delivered with the same mRNA technology that won the Nobel Prize. Then they used CAR T cell therapy to train the body’s immune system to look for that synthetic antigen and destroy the marked tumor cells.
“Tumor cells are sneaky. Most of the time, immune cells basically can’t see them because they come from our own tissues,” says Kwong, a chair and associate professor in biomedical engineering department at Georgia Tech and Emory University.
“Typically, if you’re designing a T cell to target cancers, you need to find out the things on each different cancer that you can build your T cell against. In this case, we’re designing the CAR T cell to recognize the synthetic antigen, and this becomes a universal platform.”
CAR T cell therapy has proven effective against some cancers and for some patients, especially liquid tumors such as leukemia that circulate in the blood. The patient’s own T cells, a type of white blood cell from the immune system, are engineered with a chimeric antigen receptor—that’s the CAR—and returned to the body to seek and destroy the tumors.
Unfortunately, healthy cells often end up as collateral damage because they also have the proteins the CAR T cells are looking for, creating complications for patients.
In the new study, led by then-PhD students and now research engineers Lena Gamboa and Ali Zamat, the team gave the CAR T cells a clear target that was only expressed on cancer cells and not on any healthy tissues. Their synthetic antigen is a protein not found otherwise in the body.
The combination approach also showed resilience: after the tumors were eliminated and the researchers reintroduced cancer cells, they were quickly recognized and attacked.
“We turned the tumor into a kind of immune training center so the body’s own natural immune cells could learn to recognize the cancer cells if they ever came back,” Gamboa says.
Flagging tumors this way also offers a path to treating cancers that have none of the targets that drugs can go after, giving options to patients who don’t have many options.
“Triple-negative breast cancers don’t express the typical receptors that most breast cancer therapies rely on, which leaves patients with few options beyond chemotherapy,” Zamat says.
“Instead of identifying a new drug target, we introduced one and trained the immune system to recognize the natural cancer.”
According to Gamboa, the team’s work allows them to sidestep identifying new markers to use when attacking tumors because they’re instead creating their own.
“It’s accelerating a path for us to be able to treat these cancers because now the immune system is doing the hard work of identifying what is unique about the tumor without us having to discover it first,” she says.
Kwong says they tested their therapy against breast, brain, and colon tumors to demonstrate its effectiveness on a wide range of localized solid tumors. Those also happen to be cancers known for recurring and sometimes spreading to other parts of the body. Surgically removing the tumors or treating them with radiation usually doesn’t cure the cancer. The flagging-and-attacking strategy, at least in the lab, did.
“This matters because it gives us another potential option to control local, regional disease earlier. Before it spreads. There aren’t that many options right now,” Kwong says.
“People thinking about tumor therapy tend to think about patients who are so sick that they have widely disseminated disease. But they never really ask why they have widely disseminated disease. It’s because we fail to control local disease,” he adds.
More work remains to translate the team’s synthetic antigen into an approved cancer treatment. But the team designed the approach with that in mind, Kwong says. Hundreds of thousands of patients have received other kinds of CAR T cell therapy, and it’s proven safe.
Likewise, using mRNA delivered to tumor cells via a tiny fat bubble called a lipid nanoparticle is a well understood, safe approach, Kwong says.
“We know the inflammatory profile, we know how many people have had multiple mRNA doses without any kind of adverse effects. And same for engineered CAR T cells. That makes the path to the clinic very close.”
Support for this research came from the National Institutes of Health, the National Cancer Institute, the National Institute of Biomedical Imaging and Bioengineering, the National Center for Advancing Translational Sciences, the National Science Foundation, the Shurl and Kay Curci Foundation, and the Alfred P. Sloan Foundation.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any funding agency.
Source: Georgia Tech