A major problem with understanding Alzheimer’s is not being able to clearly see why the disease starts. Now, a super-resolution “nanoscope” offers a 3D view of brain molecules with 10 times greater detail than ever before.
Recent studies show that 40 percent of Americans over the age of 85 have Alzheimer’s disease. Further, the disease begins 10 to 20 years before people ever show up at the doctor’s office with memory problems.
The instrument helped researchers better understand the structure of plaques that form in the brain of Alzheimer’s patients, pinpointing characteristics that are possibly responsible for damage.
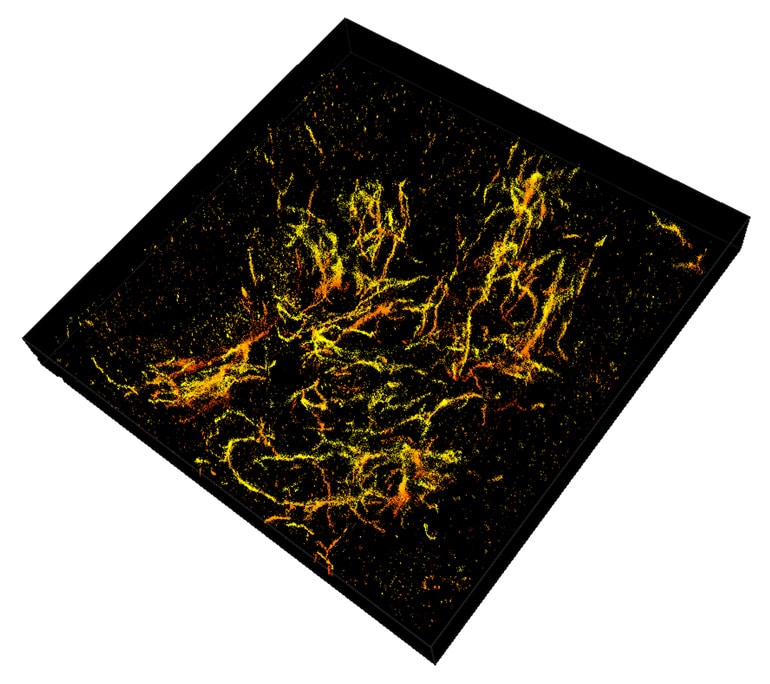
Long before Alzheimer’s develops, waxy deposits called amyloid plaques accumulate in the brain. These clusters interact with surrounding cells, causing inflammation that destroys neurons and creates memory problems.
The deposition of these plaques is currently the earliest detectable evidence of pathological change leading to Alzheimer’s disease.
“While strictly a research tool for the foreseeable future, this technology has allowed us to see how the plaques are assembled and remodeled during the disease process,” says Gary Landreth, professor of anatomy and cell biology at the Indiana University School of Medicine’s Stark Neurosciences Research Institute.
“It gives insight into the biological causes of the disease, so that we can see if we can stop the formation of these damaging structures in the brain.”
The trouble with brain tissue
The limited resolution in conventional light microscopes and the natural thickness of brain tissue have prevented researchers from clearly observing 3D morphology of amyloid plaques and their interactions with other cells.
“Brain tissue is particularly challenging for single molecule super-resolution imaging because it is highly packed with extracellular and intracellular constituents, which distort and scatter light—our source of molecular information,” says Fang Huang, assistant professor of biomedical engineering at Purdue University. “You can image deep into the tissue, but the image is blurry.”
As reported in Nature Methods, the super-resolution nanoscope, which Huang’s research team has already developed to visualize cells, bacteria, and viruses in fine detail, uses “adaptable optics”—deformable mirrors that change shape to compensate for light distortion, called “aberration,” that happens when light signals from single molecules travel through different parts of cell or tissue structures at different speeds.
How the new nanoscope works
To tackle the challenge of brain tissue, Huang’s research team developed new techniques that adjust the mirrors in response to sample depths to compensate for aberration introduced by the tissue. At the same time, these techniques intentionally introduce extra aberration to maintain the position information carried by a single molecule.
The nanoscope reconstructs the whole tissue, its cells, and cell constituents at a resolution six to 10 times higher than conventional microscopes, allowing a clear view through 30-micron thick brain sections of a mouse’s frontal cortex.
MicroRNA snippets may warn of Alzheimer’s down the road
The researchers used mice that were genetically engineered to develop the characteristic plaques that typify Alzheimer’s disease. The 3D reconstructions show that amyloid plaques are like hairballs, entangling surrounding tissue via their small fibers that branch off waxy deposits.
“We can see now that this is where the damage to the brain occurs. The mouse gives us validation that we can apply this imaging technique to human tissue,” Landreth says.
‘Fingerprint’ system could customize Alzheimer’s treatment
Researchers have already begun collaborating on using the nanoscope to observe amyloid plaques in samples of human brains, as well as a closer look at how the plaques interact with other cells and get remodeled over time.
“This development is particularly important for us as it had been quite challenging to achieve high-resolution in tissues. We hope this technique will help further our understanding of other disease-related questions, such as those for Parkinson’s disease, multiple sclerosis, and other neurological diseases,” Huang says.
Grants from the Defense Advanced Research Projects Agency, the National Institute of Health, the Indiana Clinical and Translational Sciences Institute, the National Center for Advancing Translational Sciences, the Alzheimer’s Association, the Jane and Lee Seidman Fund, the National Institute on Aging, Case Western Reserve University, and donations from Chet and Jane Scholtz and Dave and Susan Roberts funded the work.
Source: Purdue University