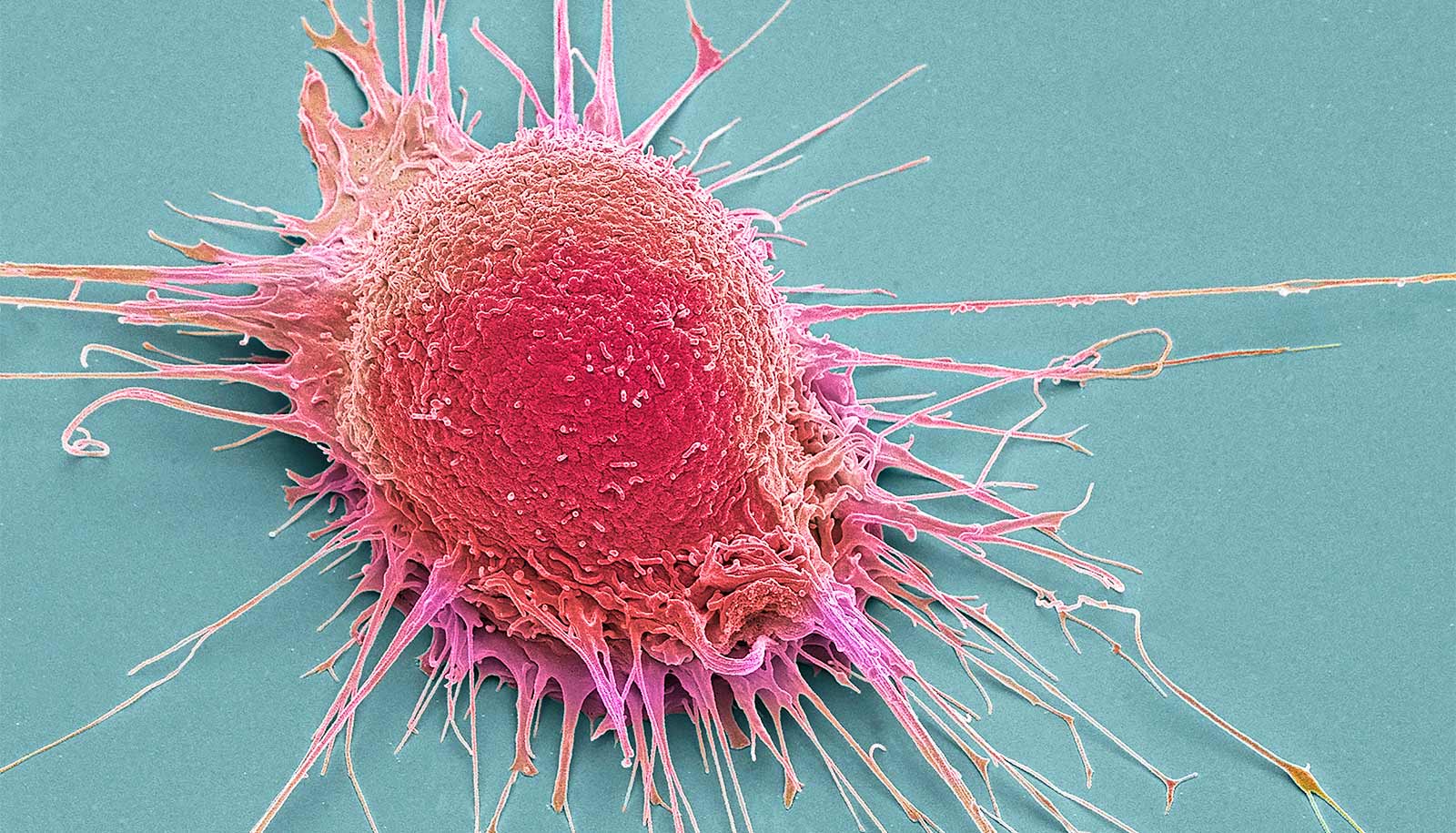
Cancer cells most often kill by crawling away from their original tumors to later re-root in vital parts of the body in a process called metastasis.
Bristly leg-like protrusions that cover cancer cells enable them to creep. When researchers gently heated minuscule gold rods with a laser in experiments on common laboratory cultures (in vitro) of cancerous human cells, they were able to mangle the protrusions and prevent cell migration, a key mechanism in metastasis.
In the future, the technique could potentially offer clinicians going after individual tumors a weapon to combat cancer’s spread at the same time.
“If cancer stays in a tumor in one place, you can get to it, and it’s not so likely to kill the patient, but when it spreads around the body, that’s what really makes it deadly,” says lead researcher Mostafa El-Sayed, professor of chemistry and biochemistry at Georgia Institute of Technology and lead researcher of the study in the Proceedings of the National Academy of Sciences.
Squished cancer cells break free from the crowd
The treatment can also easily kill cancer cells, but in this experiment, it was vital to specifically show that it greatly slowed cell migration.
The experimental treatment also spares healthy cells, in these and in prior experiments, making it potentially much less taxing on patients than commonly used chemotherapy. In past tests in animal models, there were no toxic side effects from the gold used in the treatment, and no observable damage to healthy tissue from the low-energy laser. And there was no recurrence of the treated cancer.
“The method appears to be very effective as a locally administered treatment that also protects the body from cancers spread away from the treated tumors, and it is also very mild, so it can be applied many times over if needed,” El-Sayed says.
How it works
A close-up look at a cell and what malignant cancer can do to it clarifies how the treatment works, researchers say.
Many people think of cells as watery balloons—fluid encased in a membrane sheath with organelles floating around inside. But that picture is incomplete. Cells have support grids called cytoskeletons that give them form and that have functions.
The cytoskeletons also form bristly protrusions called filopodia, which extend out from a weave of fibers called lamellipodia on the cells fringes. The protrusions normally help healthy cells shift their location in the tissue that they are part of.
But in malignant cancer, normally healthy cell functions often lunge into destructive overdrive. Lamellipodia and filopodia are wildly overproduced.
“All these lamellipodia and filopodia give the cancer cells legs,” says Yue Wu, a graduate student in bioanalytical chemistry. “The metastasis requires those protrusions, so the cells can travel.”
Bubble pushes 1 cancer cell from device at a time
The gold nanorods thwart the protrusions in two ways. The rods are comprised of a small collection of gold atoms—nano refers to something being just billionths of meters (or feet) in size.
First, El-Sayed’s nanorods are introduced locally, where they encumber the leggy protrusions on cancerous cells. The rods are coated with molecules (RGD-peptides) that make them stick specifically to a type of cell protein called integrin.
“The targeted nanorods tied up the integrin and blocked its functions, so it could not keep guiding the cytoskeleton to overproduce lamellipodia and filopodia,” says Yan Tang, a postdoctoral assistant in computational biology. The binding of the integrin alone slowed down the migration of malignant cells.
But healthy cells stayed safe. “There are certain, specific integrins that are overproduced in cancerous cells,” says Moustafa Ali, one of the study’s first authors. “And you don’t find them so much in healthy cells.”
Gentle laser
In the second phase, researchers hit the gold nanoparticles with a low-energy laser of near-infrared (NIR) light. It brought the migration of the cancer cells to an observable halt.
“The light was not absorbed by the cells, but the gold nanorods absorbed it, and as a result, they heated up and partially melted cancer cells they are connected with, mangling lamellipodia and filopodia,” Ali says. “It didn’t kill all the cells, not in this experiment. If we killed them, we would not have been able to observe whether we stopped them from migrating or not.” But if necessary, the treatment can be adjusted to kill the cells.
Early experiments in animal models in vivo with hotter lasers didn’t work as well. “That caused inflammation, which made it possible to heat one time only,” Ali says. “As a result, that high temperature would wipe out many cancer cells, but not all of them. Some hidden ones might have survived, and also still been able to migrate.”
“This gentle laser didn’t burn the skin or damage tissue, so it could be dosed multiple times and more thoroughly stop the cancer cells from being able to travel,” says researcher Ronghu Wu.
How some breast cancer cells return after chemo
Researchers envision treating head, neck, breast, and skin cancers with direct, local nanorod injections combined with the low-power near-infrared laser, which can hit the gold nanorods 2-3 centimeters (around an inch) deep inside tissue. “But it could go as deep as 4-5 centimeters,” Ali says.
Deeper tumors could conceivably be treated with deeper injections of nanorods. “Then you’d need to go in with a fiber optic or endoscopic laser,” El-Sayed says. Injecting the nanorods directly into the bloodstream as a broad treatment is not currently a viable option.
El-Sayeds group has previously published in vivo experiments in mice in the Proceedings of the National Academy of Sciences together with Emory University School of Medicine. That study showed no observable toxicity from the gold in mice 15 months after treatment.
“A lot of it ended up in the liver and spleen,” El-Sayed says. “But the functions of these organs appeared intact upon examination, and treated mice were alive and healthy over a year later.”
Other coauthors are from Georgia Tech and Georgia State University. The National Science Foundation Division of Chemistry and the National Institutes of Health funded the work.
Source: Georgia Tech