Researchers have created a new material that allows the effective delivery of both chemotherapy drugs and gene therapy designed to fight drug resistance.
Clinicians today have an arsenal of more than 200 drugs at their disposal for treating a range of cancers—68 drugs were approved between 2011 and 2016 alone. Many chemotherapeutic agents pose stubborn challenges, however:
- they cause serious side effects because they kill healthy cells in addition to cancer cells;
- some forms of cancer develop resistance to drugs;
- and many such chemotherapies, being poorly water-soluble, demonstrate low bio-availability resulting in sub-optimal drug delivery to cancer cells.
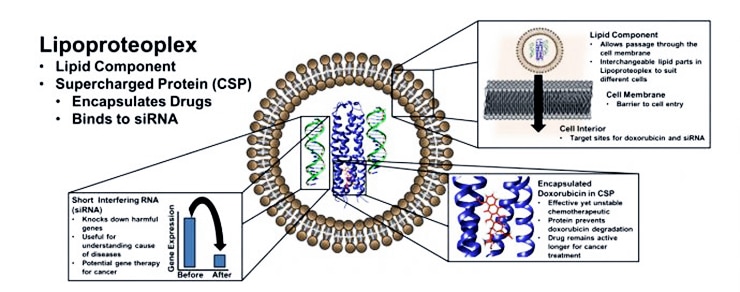
A potential solution lies in the synergistic combination of a chemotherapeutic drug with engineered genetic material designed to neutralize the malevolent genes conferring resistance to that drug, among other functions.
While there are numerous examples of synthetic dual gene and drug delivery vehicles, the new hybrid materials use easily modifiable proteins to deliver a chemical one-two punch: they combine a lipid “container” for transfection—the transportation of cargo past a cell membrane—and an easy-to-make protein capsule that can bind both small chemotherapeutic molecules and nucleic acids.
The hybrid lipid-protein material, called a lipoproteoplex, comprises both a coiled supercharged protein macromolecule and a commercially available transfection agent called Lipofectamine 2000.
Because the researchers engineered the protein macromolecule with extensive positive charges on the surface and a hydrophobic core, it can be easily festooned with negatively charged short interfering RNA (siRNA)—a powerful tool for suppressing genes that invoke drug resistance and propagate disease states—while also serving as an efficient, and toxicity reducing, carryall for the hydrophobic chemotherapeutic agent doxorubicin.
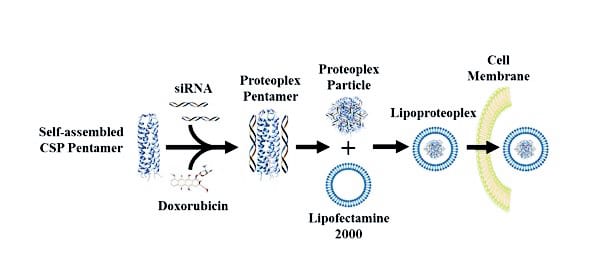
In research published in Biomacromolecules, the team details how the lipoproteoplex exposed to samples of the MCF-7 breast cancer cell line delivered more doxorubicin to target cells than did Lipofectamine 2000 alone, resulting in a substantial decrease in MCF-7 cell viability. They also demonstrated that the hybrid macromolecule was highly successful at siRNA transfection, silencing the gene by 60 percent.
Jin Kim Montclare, associate professor of chemical and biomolecular engineering at New York Univeristy’s Tandon School of Engineering, says a key benefit of the new lipoproteoplex is ease of modification, an asset to researchers studying cells whose genetically invoked behavior changes over time and differs by cell line and patient.
Rather like a system of mix-and-match components, the lipoproteoplex allows researchers to swap out a supercharged protein or lipid component and any number of siRNA to address a specific cell line and type of drug.
Light and B12 let red blood cells deliver drugs
“Unlike other pursuits at producing dual gene and drug delivery systems, this approach doesn’t require tedious chemical synthesis procedures; rather we can biosynthesize any variant of the supercharged protein,” says Montclare. “This allows for substituting different siRNA molecules and chemotherapeutic drugs to suit lab needs.”
In recent work on protein-based hybrid materials, Montclare and her collaborators combined an engineered supercharged protein with the transfection reagent Fugene. The combination exhibited an eightfold improvement in transfection efficiency of DNA compared to Fugene alone, with negligible cytotoxicity.
Montclare is investigating the mechanisms that enable these lipoproteoplexes to effectively deliver genes and drugs across different cell lines.
Additional researchers who contributed to this work are from New York University, the State University of New York Downstate Medical Center, and the Simons Foundation’s Flatiron Institute Center for Computational Biology.
DNA ‘cages’ deliver drugs with zap of light
The National Science Foundation, the National Center for Advancing Translational Sciences, and the National Institutes of Health supported this research with grants.
Source: New York University