A new system can convert stem cells to target cells and then remove the remnants of the conversion, leaving only the desired DNA behind to duplicate.
“One difficulty with human pluripotent stem cells is that you can’t use them directly,” says Xiaojun Lian, assistant professor of biomechanical engineering and biology and a member of the Huck Institutes of the Life Sciences at Penn State. “They need to be functional cell types. If you have a heart attack you want cells that will repair the heart wall.”
Normally, pluripotent stem cells induced from both adult and embryonic cells receive a chemical signal to change from a stem cell to a functional cell. Pluripotent stem cells can change to any cell in the human body. However, this natural cell change is part of a complex series of triggers controlled by DNA.
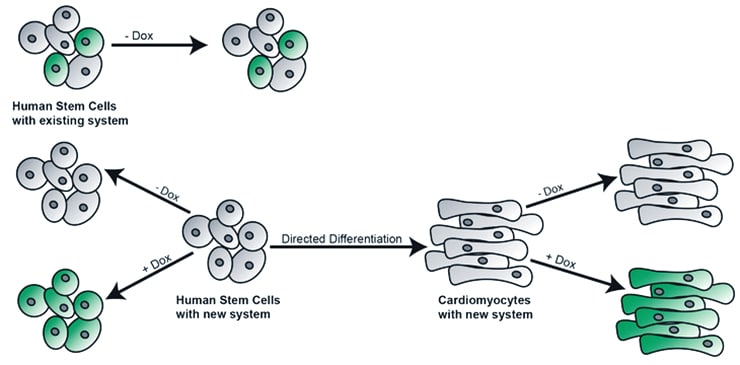
Researchers have in the past inserted DNA into the pluripotent cells to convert them, but remnants of the inserted DNA remain.
In this current work, published in a recent issue of Scientific Reports, the researchers are not incorporating a piece of DNA that will tell the cells to convert, but DNA that will make the cell glow green when illuminated by a blue light. This marker allows the researchers to see that the DNA plasmid is incorporated into the cell, and that it is completely gone upon removal.
A plasmid is a circular piece of DNA that contains functional DNA fragments that control gene expression in cells.
“We wanted to explore the limits for turning the conversion on and off and to have the ability to control the level of expression and removal of DNA after conversion,” says Lauren N. Randolph, a doctoral student in bioengineering.
Previous approaches incorporated the appropriate DNA to switch on the conversions, but did not completely remove all the DNA inserted.
The researchers are using a Tet-On 3G inducible PiggyBac system that is a plasmid they named XLone to achieve insertion, activation, and removal. The PiggyBac portion of the system includes the DNA to insert that DNA into the cell’s DNA. The Tet-On 3G portion contains the necessary signaling information. This system also makes the cells more sensitive to doxycycline, which is the drug used to initiate the conversion.
How inkjet printers help transform stem cells
“We are using abundant multiple copies of the plasmid to increase the likelihood that it gets in and does what it is supposed to do and actually follows through reproduction of the cells,” says Lian.
If only one or a few plasmids are inserted into the cell, the new DNA could just be silenced. Insertion of multiple plasmids assures that at least one will function.
“The first advantage with our system is that it does not have any leakage expression,” says Randolph. “If we don’t induce the system with doxycycline, we get nothing.”
How to reprogram stem cells into motor neurons
The second advantage is that once the cells are reproducing as heart cells or nerve cells, the plasmid can be removed and the cells continue to reproduce without any remnant of the plasmid system.
While the researchers are currently aiming to understand and study gene function and directed cell differentiation in human stem cells, eventually they would like to be able to create cell-based therapies for a range of degenerative diseases.
Others working on this project are from the University of California, Berkeley and the Karolinska Institute in Sweden. The Penn State Bioengineering Department, Biology Department, and Huck Institutes of the Life Sciences supported this work.
Source: Penn State