A new study finds it’s possible to target stem cells for protective vaccination from tuberculosis, but that a pathogen can also hijack them to increase TB virulence.
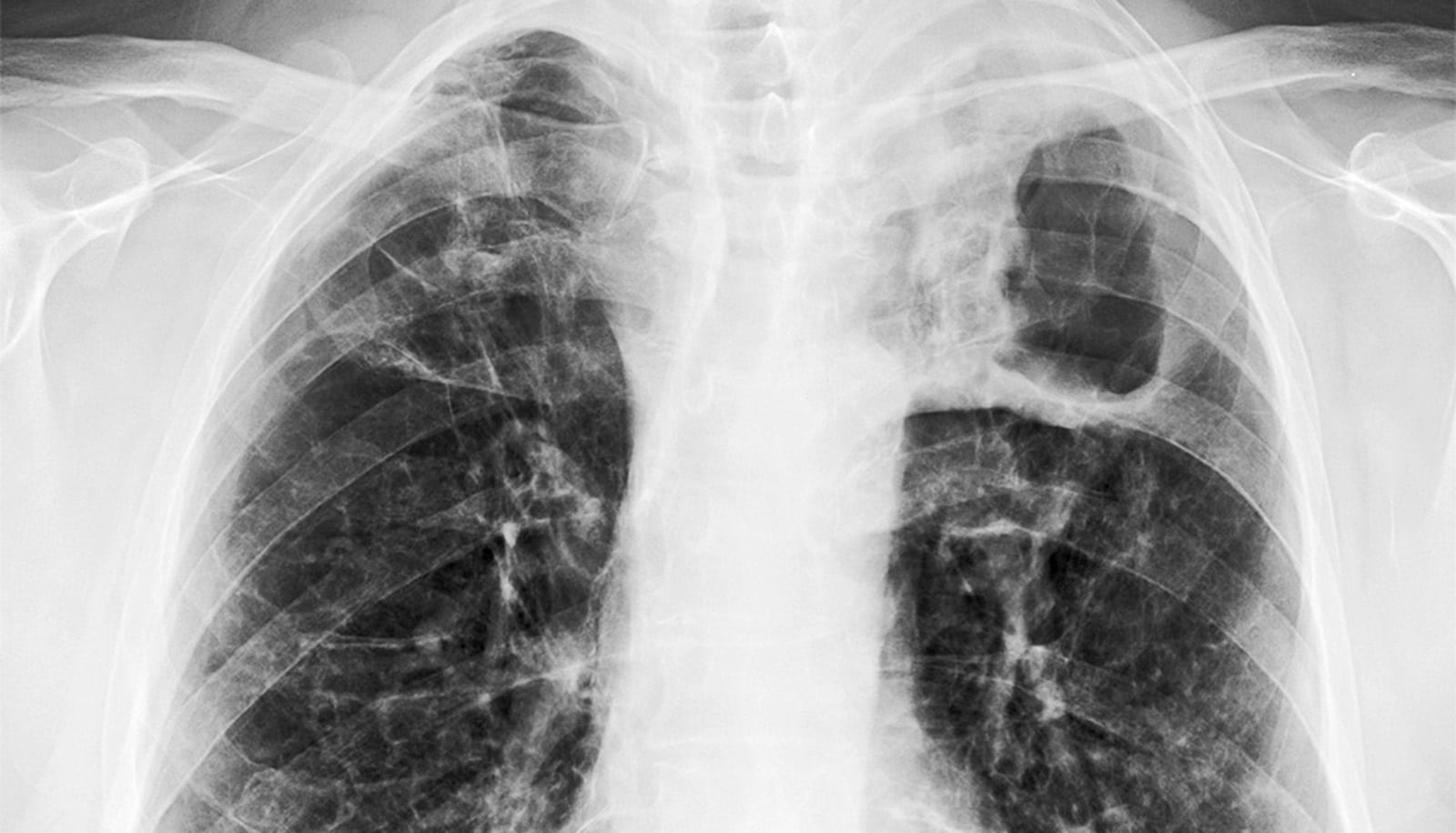
Since Robert Koch discovered Mycobacterium tuberculosis (Mtb)—the causative agent of tuberculosis—in the 19th century, TB has defied efforts by scientists to end this old pandemic that still kills around 1.6 million people annually. In fact, TB still kills one person every 22 seconds.
A team of researchers had previously shown that exposure of bone marrow stem cells—
responsible for generating all the immune cells—to a live BCG vaccine (the only available vaccine for TB) reprograms these cells to generate protective innate immunity against TB. However, the question remained: What are the consequences of stem cell exposure to the pathogen Mtb?
The question gets an answer in a paper in the journal Cell. The team demonstrates in mice that at a very early stage (7-10 days) of pulmonary Mtb infection, bacteria move from the lungs into the bone marrow and reprogram stem cells to impair innate immunity against TB. The study also demonstrates that both the protective impact of BCG and the detrimental effect of virulent Mtb on immunity last for at least a year.
“What’s new in this study is that we now know that Mtb hijacks the immune response at the very early phase of infection by accessing the bone marrow and manipulating stem cells. This leads to the generation of impaired innate immune cells, which are effectively incapacitated to fight the infection in the lung, thus allowing the bacteria to grow,” says Nargis Khan, first author of the study and a member of the lab of study co-leader Maziar Divangahi, pulmonary immunologist at the Research Institute of the McGill University Health Centre (RI-MUHC) and a professor of medicine at McGill University.
Stem cells are usually in dormancy but “wake up” when there is demand, such as following stress or infection. They rapidly adapt to the threat by generating all immune cells and can be “educated” to produce innate immune cells that confer increased protection against infection—a concept called trained immunity.
Focus on bone marrow, not lungs
The findings reveal the molecular mechanism by which Mtb-exposed stem cells reduce both the number and the anti-microbial capacity of macrophages—the white blood cells that normally kill invading bacteria—which are also the immune system’s first responders.
“Mechanistically, we uncovered that regulation of iron levels within stem cells is critical in maintaining their ability to generate protective macrophages, and those levels were altered by the presence of Mtb,” says Jeffrey Downey, co-first author of the study and PhD candidate in Divangahi’s laboratory.
“Iron is an essential micronutrient, required by both humans and Mtb to survive, thus, this manipulation of iron levels in stem cells by Mtb represents an interesting new link between nutrient acquisition and pathogen virulence.”
“Eventually, the immune cells need to go to the site of infection and fight the pathogen,” explains Divangahi. “But once the function of the stem cells which are responsible for generating them has been corrupted by Mtb, they lose their ability to fight effectively against the infection in the lung.
“Instead of focusing on the lung as the initial site of many pulmonary infections,” he adds, “it will be better to crack the protective code of stem cells in the bone marrow—the privileged site that is responsible for generating the entire immune cells against all invaders.”
From TB to SARS-CoV-2?
Given the lack of targeted therapy or vaccine, alternative approaches to eradicate TB are urgently needed. Understanding how to harness the power of innate immunity is a promising novel avenue, not only for TB, but for other infectious diseases like COVID-19.
Based on this approach, scientists could potentially develop vaccines designed to provide broad protection against many infectious diseases. Some are already testing the efficiency of existing live vaccines to fight other infections.
“This is the rationale behind various clinical trials happening worldwide to determine if the BCG vaccine can enhance the innate immune system against SARS-CoV-2, the virus responsible for COVID-19,” says Divangahi.
“Although we are certainly in the infancy of understanding trained immunity, we are working to bridge the knowledge gap between what we see in the laboratory and what we can use in patients.”
Studies like this, and future ones also aimed at understanding the mechanisms of trained immunity in the context of a variety of infections, are needed to achieve this goal.
The Canadian Institutes of Health Research funded the work.
Source: McGill University