Some human cells use a slingshot motion to move forward and travel five times faster than previously documented, according to a new study.
Researchers observed this for the first time in bioengineered 3D scaffolds that model stromal tissue—the connective tissue that surrounds organs—and say the spread of cancer could involve this type of cell movement.
Further, the researchers say they could potentially harness the cell movement method in the future to direct the movement of healthy cells for tissue repair therapies.
The cells pull on and stretch the surrounding fibrous tissue, eventually using that tension to launch themselves forward. The motion looked familiar enough that researchers dubbed it “slingshot migration.”
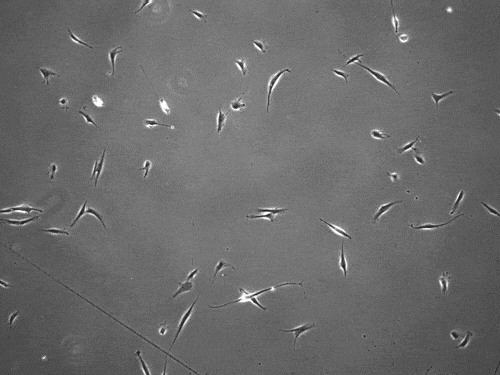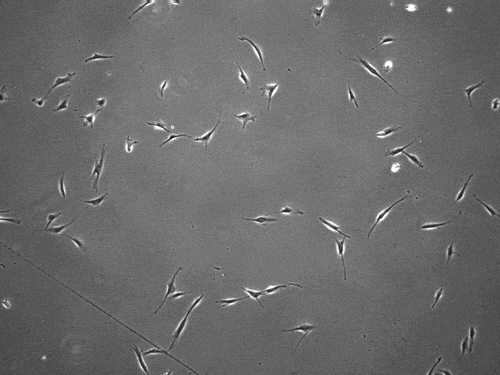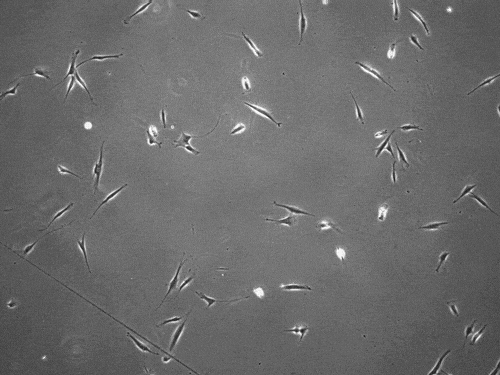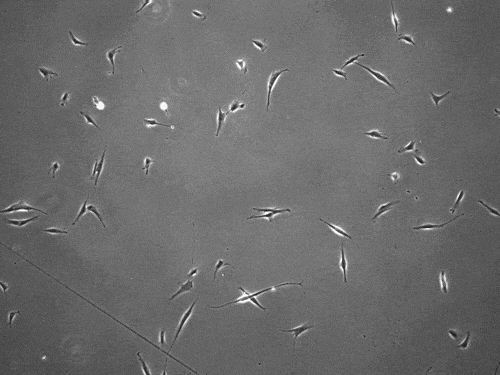
“We found that cells can move in this very distinct way that results in effective migration far faster than anything previously reported,” says Brendon Baker, assistant professor of biomedical engineering at the University of Michigan.
William Wang, a doctoral student in biomedical engineering, witnessed it first while studying the properties of stromal tissue that either hinder or promote the spread of cells in diseases such as cancer.
“I was definitely shocked seeing a cell move so fast,” Wang says. “What was even more surprising was then capturing this migration mode in multiple cell types and finding that their speed was so much faster than traditional modes of cell migration.”
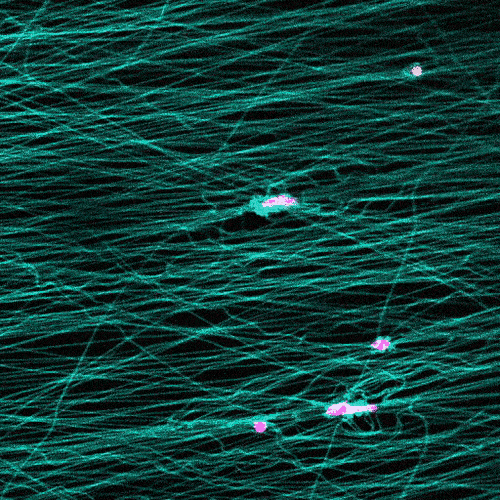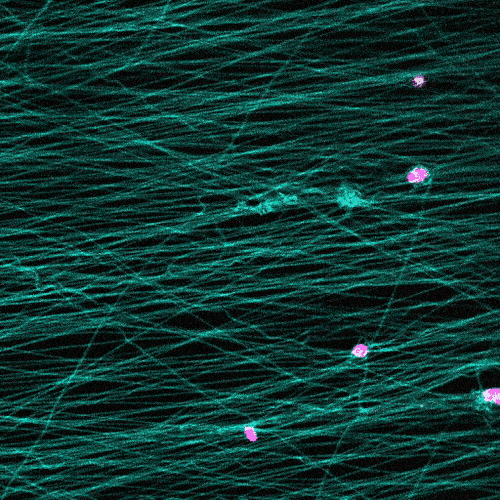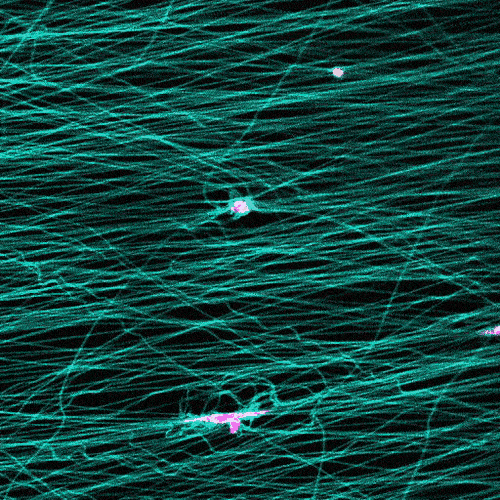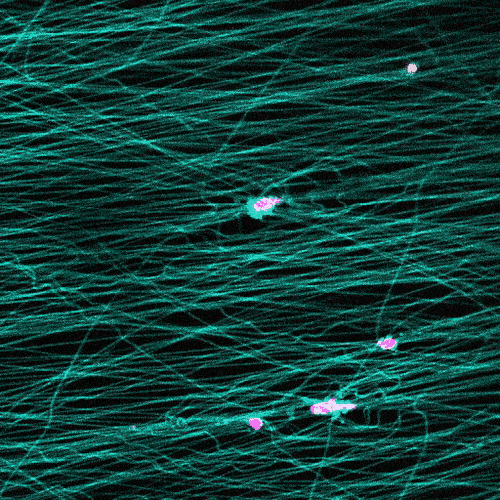
Research in this area typically involves observing cells under the microscope on a flat petri dish. But that doesn’t provide a complete picture.
“These flat, two-dimensional surfaces really don’t resemble the structure and mechanical behavior of real tissues, especially the ones where so much of the migration in our body is occurring,” Wang says.
The researchers study cells in a 3D fibrous environment, closer to the real thing.
Most organs can be broken down into two parts: functional elements such as sacs, ducts, or glands called the parenchyma, and the surrounding collagenous tissue that supports the blood vessel network, called the stroma or interstitia.
Baker’s lab builds stromal tissue for two applications: to study how cells behave during disease progression, and to advance organ replacement therapies.
Surgical removal can usually deal with cancer cells that remain within the parenchyma, where most originate, researchers say. When they migrate into the stromal tissue, they pose a much greater threat.
“It’s metastasis, or the spread of the cancer, that kills patients,” Baker says. “For that to happen, the cells have to break out of the parenchyma and cross through the fibrous stroma to reach blood or lymphatic vessels or the lymphatic system.”
The National Institutes of Health funded the research, which appears in Nature Communications.
Source: University of Michigan



