Researchers have developed a new kind of polymer that can capture anesthetic gases, possibly making way for the gases to be recycled.
Recycling anesthetic gases could cut down on harm to the environment they cause and potentially reduce surgery costs.
Every year more than 300 million major operations take place in hospitals around the world. Each has the potential to greatly improve or even save a life, yet many rely on anesthesia gases that, as well as making patients unconscious, contribute to climate change—some are thousands of times more potent than carbon dioxide.
When you consider that a busy, mid-sized hospital has been estimated to produce greenhouse gases equivalent to up to 1,200 cars, “the global warming potential is considerable,” says Brendan Abrahams, associate professor at the University of Melbourne.
And it’s not only the environment that’s at risk. During surgical anesthesia, some of these gases can leak into operating rooms and veterinary clinics where, over time, they may pose health risks (occupational exposure to anesthetic gases has been linked to inflammatory responses in staff).
But the vast majority of these gases are expelled from patients’ lungs and flushed outside into the atmosphere where they can trap heat.
Imagine, then, that these gases could be somehow captured, stored, and maybe even recycled? Besides helping tackle global warming and the health of workers, it could possibly reduce surgical costs, researchers say.
The researchers believe that the technology could not only capture potentially damaging gases, but could also be used to harvest from patients’ lungs one of the cleanest, rarest, and safest anesthetic gases known to medicine, xenon—a gas that has no known impact on either the environment or surgical staff.
This is one of those serendipitous science stories that began not with a problem looking for an answer, but with an answer just waiting for the right problem to come along.
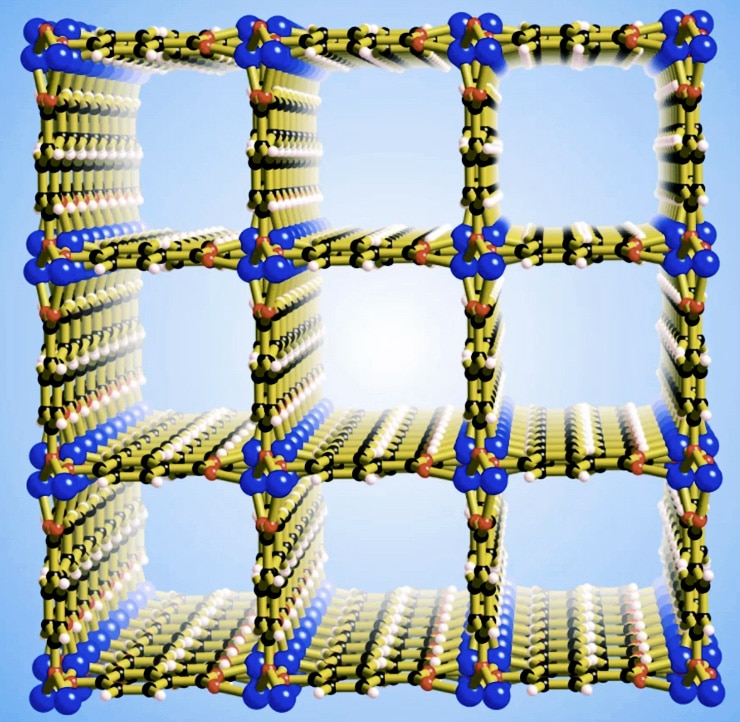
The groundwork was laid in the late 1980s when University of Melbourne chemist Professor Richard Robson pioneered a new technology that allowed scientists to design and generate materials known as coordination polymers, which contain holes large enough to hold small molecules.
Zoom in and what you see is something that might more closely resemble an IKEA storage cabinet.
The inspiration for the research came from the construction of molecular models which Robson designed for use in undergraduate chemistry lectures in the 1970s.
To the naked eye, it looks a little like white crystalline powder or perhaps fine sand. But zoom in and what you see is something that might more closely resemble an IKEA storage cabinet—a sort of molecular scaffolding.
The porous structures have since been deployed for uses including storing gaseous fuels (such as methane), and purifying chemical compounds. Many have also shown unusual and possibly useful magnetic and electronic properties.
Fast forward 20 years or so to the spring of 2012. Abrahams is having coffee with a colleague, chatting about coordination polymers that the Robson-Abrahams team have already created to see if they can be used to separate and store CO2 molecules (it turns out they can, but not at commercially viable prices).
Can anesthesia protect lungs from the flu?
Then Abrahams’ colleague, Paul Donnelly, had another idea. Part of his own research involves anesthetizing mice. Could the technology be repurposed?
The team has since developed a new family of “tuneable” polymers that can be tailored to fit individual anesthetic gases. Big holes for big molecules, snugger holes for smaller ones.
The researchers have managed to capture and store two commonly-used inhalation anesthetics, isoflurane and sevoflurane. They now plan to turn their attention to another popular vapor, desflurane. They’ve already established that once the gases are captured, the coordination polymers can be reheated slightly until they release the vapor into an apparatus where it then cools down and condenses back into liquid form.
Ideally, the next step is to see if the gases can be recycled (the molecules are just molecules, so it makes no difference how many sets of lungs they pass through), reducing waste and costs, Abrahams says.
Then there is xenon. Considered by some the perfect anesthetic, this inert gas is non-explosive, non-flammable, relatively non-toxic—and doesn’t damage the environment. The problem is that it’s rare—87 parts per billion—and expensive. Researchers have managed to capture xenon with their coordination polymers, and hope this might eventually boost the gas’s use in operating rooms.
“If you could capture all the xenon back from the patient, then it may become economically viable,” Abrahams says. “We’re hopeful that this basic chemical research might translate into a commercially viable and environmentally helpful product.”
A paper outlining the research appears in in the journal Chemistry.
Source: Kate Cole-Adams for University of Melbourne