A soft, implantable device can detect over-activity in the bladder and then use light from tiny, biointegrated LEDs to tamp down the urge to urinate.
The device works in laboratory rats and could one day help people who suffer incontinence bypass the need for medication or electronic stimulators, researchers say.
Overactive bladder, pain, burning, and a frequent need to urinate are common and distressing problems. For about 30 years, stimulators that send an electric current to the nerve that controls the bladder were the treatment for patients with severe bladder problems. Such implants improve incontinence and overactive bladder, but they also can disrupt normal nerve signaling to other organs.
“There definitely is benefit to that sort of nerve stimulation,” says Robert W. Gereau IV, professor of anesthesiology at Washington University in St. Louis School of Medicine. “But there also are some off-target side effects that result from a lack of specificity with those older devices.” Researchers developed the device in hopes of preventing these kinds of side effects.
Bluetooth communication
During a minor surgical procedure, the team implanted a soft, stretchy, belt-like device around the bladder. As the bladder fills and empties, the belt expands and contracts. The researchers also injected proteins called opsins into the animals’ bladders.
A virus that binds to nerve cells in the bladder carries the opsins, making them sensitive to light signals. This allowed the researchers to use optogenetics—the use of light to control cell behavior in living tissue—to activate those cells.
Using Bluetooth communication to signal an external hand-held device, the scientists can read information in real time and, using a simple algorithm, detect when the bladder is full, when the animal has emptied its bladder, and when the bladder is emptying too frequently.
“When the bladder is emptying too often, the external device sends a signal that activates micro-LEDs on the bladder band device, and the lights then shine on sensory neurons in the bladder,” Gereau says. “This reduces the activity of the sensory neurons and restores normal bladder function.”
As needed basis
The researchers believe a similar strategy could work in people. Devices for people likely would be larger than the ones used in rats. Researchers could implant them without surgery, using catheters to place them through the urethra into the bladder.
“We’re excited about these results,” says John A. Rogers, professor of materials science and engineering, biomedical engineering, and neurological surgery at Northwestern University.
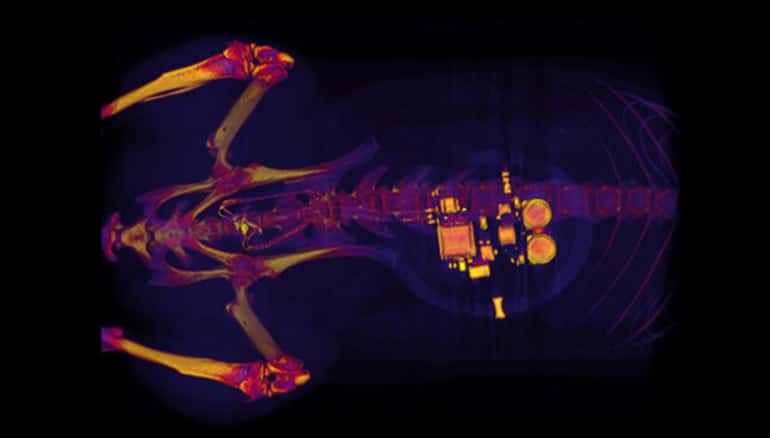
“This example brings together the key elements of an autonomous, implantable system that can operate in synchrony with the body to improve health: a precision biophysical sensor of organ activity; a noninvasive means to modulate that activity; a soft, battery-free module for wireless communication and control; and data analytics algorithms for closed-loop operation.”
Closed-loop operation essentially means the device delivers the therapy only when it detects a problem. When the behavior is normalized, the micro-LEDs are turned off, and therapy can stop.
The researchers expect to test similar devices in larger animals and believe the strategy could treat other parts of the body—for chronic pain for example, or using light to stimulate cells in the pancreas to secrete insulin. One hurdle, however, involves the viruses used to get light-sensitive proteins to bind to cells in organs.
“We don’t yet know whether we can achieve stable expression of the opsins using the viral approach and, more importantly, whether this will be safe over the long term,” Gereau says. “That issue needs to be tested in preclinical models and early clinical trials to make sure the strategy is completely safe.”
Additional researchers are from Washington University in St. Louis, Northwestern, and the University of Illinois in Urbana-Champaign. The National Institutes of Health and the Dominantly Inherited Alzheimer’s Network funded the work.