Researchers have developed a method for delivering cancer-killing drugs inside tumors with gold nanoparticles they can activate remotely using a laser.
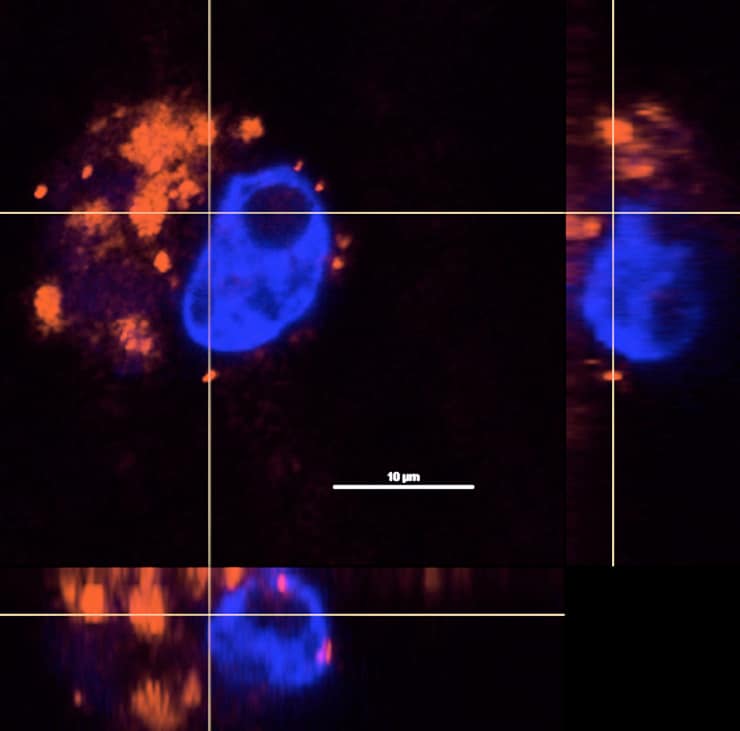
The researchers employed gold nanoshells to deliver toxic doses of two drugs—lapatinib and docetaxel—inside breast cancer cells, showing they could use a laser to remotely trigger the particles to release the drugs after they entered the cells.
Though researchers conducted the tests with cell cultures in a lab, they designed the research to demonstrate clinical applicability: The nanoparticles are nontoxic, the drugs are widely used, and the low-power, infrared laser can noninvasively shine through tissue and reach tumors several inches below the skin.
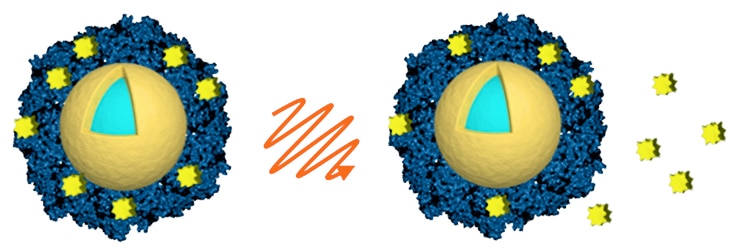
“In future studies, we plan to use a Trojan-horse strategy to get the drug-laden nanoshells inside tumors,” says Naomi Halas, an engineer, chemist, and physicist at Rice University who invented gold nanoshells and has spent more than 15 years researching their anticancer potential.
“Macrophages, a type of white blood cell that’s been shown to penetrate tumors, will carry the drug-particle complexes into tumors, and once there we use a laser to release the drugs.”
Taking on tumors
Coauthor Susan Clare, a research associate professor of surgery at the Feinberg School of Medicine at Northwestern University, says the study was designed to demonstrate the feasibility of the Trojan-horse approach. In addition to demonstrating that drugs could be released inside cancer cells, the study also showed that, in macrophages, the drugs did not detach prior to triggering.
“Getting chemotherapeutic drugs to penetrate tumors is very challenging,” says Clare, also a breast cancer surgeon. “Drugs tend to get pushed out of tumors rather than drawn in. To get an effective dose at the tumor, patients often have to take so much of the drug that nausea and other side effects become severe.
“Our hope is that the combination of macrophages and triggered drug-release will boost the effective dose of drugs within tumors so that patients can take less rather than more,” she says.
If the approach works, Clare says, it could result in fewer side effects and potentially treat many kinds of cancer. For example, one of the drugs in the study, lapatinib, is part of a broad class of chemotherapies called tyrosine kinase inhibitors that target specific proteins linked to different types of cancer.
Other Federal Drug Administration-approved drugs in the class include imatinib (leukemia), gefitinib (breast, lung), erlotinib (lung, pancreatic), sunitinib (stomach, kidney), and sorafenib (liver, thyroid, and kidney).
“All the tyrosine kinase inhibitors are notoriously insoluble in water,” says Amanda Goodman, lead author of the study. “As a drug class, they have poor bioavailability, which means that a relatively small proportion of the drug in each pill is actually killing cancer cells. If our method works for lapatinib and breast cancer, it may also work for the other drugs in the class.”
Spheres of glass and gold
Halas invented nanoshells in the 1990s. About 20 times smaller than a red blood cell, they are made of a sphere of glass covered by a thin layer of gold. Nanoshells can be tuned to capture energy from specific wavelengths of light, including near-infrared (near-IR), a nonvisible wavelength that passes through most tissues in the body.
Nanospectra Biosciences, a licensee of this technology, has performed several clinical trials over the past decade using nanoshells as photothermal agents that destroy tumors with infrared light.
Clare and Halas’ collaboration on nanoshell-based drug delivery began more than 10 years ago. In earlier work, they showed that a near-IR continuous-wave laser—the same kind that produces heat in the photothermal applications of nanoshells—could be used to trigger the release of drugs from nanoshells.
Could engineered proteins deliver chemo to tumors?
In the latest study, Goodman contrasted the use of continuous-wave laser triggering and triggering with a low-power pulse laser. Using each type of laser, she demonstrated the remotely triggered release of drugs from two types of nanoshell-drug conjugates. One type used a DNA linker and the drug docetaxel, and the other employed a coating of the blood protein albumin to trap and hold lapatinib.
In each case, Goodman found she could trigger the release of the drug after the nanoshells were taken up inside cancer cells. She also found no measurable premature release of drugs in macrophages in either case.
Halas and Clare says they hope to begin animal tests of the technology soon and have an established mouse model that could be used for the testing.
“I’m particularly excited about the potential for lapatinib,” Clare says. “The first time I heard about Naomi’s work, I wondered if it might be the answer to delivering drugs into the anoxic (depleted of oxygen) interior of tumors where some of the most aggressive cancer cells lurk.
“As clinicians, we’re always looking for ways to keep cancer from coming back months or years later, and I am hopeful this can do that,” she says.
‘Universal cassette’ modifies antibodies for drug delivery
The study appears in the Proceedings of the National Academy of Sciences.
Additional coauthors of the paper are from Rice University, Northwestern University, and the University of Copenhagen. The Department of Defense Breast Cancer Research Program, the Welch Foundation, and the National Science Foundation supported the research.
Source: Rice University