A new way to make arrays of nanofibers that gets its inspiration from polar bear fur, lotus leaves, and gecko feet could lead to coatings that are sticky, repellant, insulating, or light emitting.
“This is so removed from anything I’ve ever seen that I would have thought it was impossible,” says Joerg Lahann, a professor of chemical engineering at the University of Michigan and senior author of the paper, which appears in Science.
Polar bear hairs are structured to let light in while keeping heat from escaping. Water-repelling lotus leaves are coated with arrays of microscopic waxy tubules. And the nanoscale hairs on the bottoms of gravity-defying gecko feet get so close to other surfaces that atomic forces of attraction come into play.
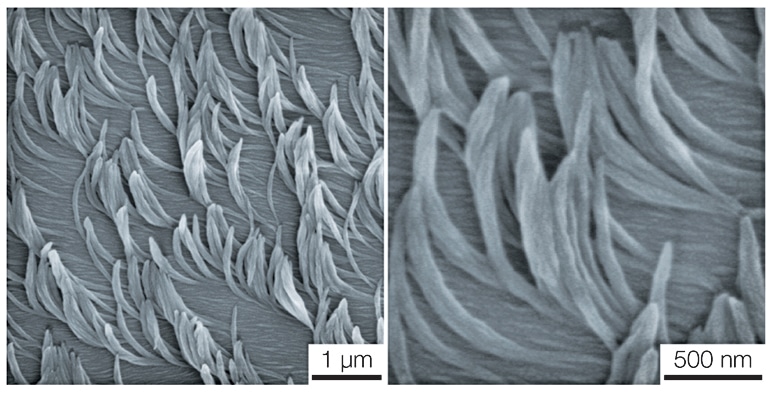
Researchers looking to mimic these superpowers needed a way to create the minuscule arrays that do the work. “Fundamentally, this is a completely different way of making nanofiber arrays,” Lahann says.
Repel, stick, insulate
The researchers have shown that their nanofibers—which are hundreds of times thinner than a human hair—repelled water like lotus leaves. They grew straight and curved fibers and tested how they stuck together like Velcro—finding that clockwise and counterclockwise twisted fibers knitted together more tightly than two arrays of straight fibers.
They also experimented with optical properties, making a material that glows—and believe it will be possible to make a structure that works like polar bear fur, with individual fibers structured to channel light.
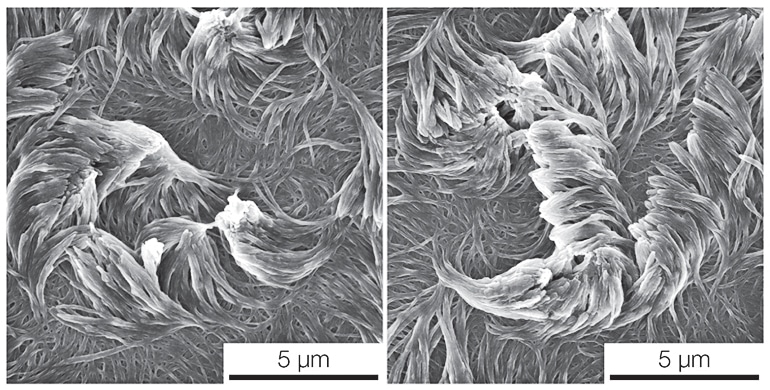
But molecular carpets weren’t the original plan. Lahann’s group was working with Nicholas Abbott, at the time a professor of chemical engineering at the University of Wisconsin-Madison, to put thin films of chain-like molecules, called polymers, on top of liquid crystals.
Television and computer screen displays use liquid crystals. The researchers were trying to make sensors that could detect single molecules.
“The discovery reinforces my view that the best advances in science and engineering occur when things don’t go as planned.”
Lahann brought the expertise in producing thin films and Abbott led the design and production of the liquid crystals. In typical experiments, Lahann’s group evaporates single links in the chain and coaxes them to condense onto surfaces.
But the thin polymer films sometimes didn’t materialize as expected.
“The discovery reinforces my view that the best advances in science and engineering occur when things don’t go as planned,” Abbott says. “You just have to be alert and view failed experiments as opportunities.”
‘Like microscopic bananas’
Instead of coating the top of the liquid crystal, the links slipped into the fluid and connected with each other on the glass slide. The liquid crystal then guided the shapes of the nanofibers growing up from the bottom, creating nanoscale carpets.
“A liquid crystal is a relatively disordered fluid, yet it can template the formation of nanofibers with remarkably well-defined lengths and diameters,” Abbott says.
And they didn’t only make straight strands. Depending on the liquid crystal, they could generate curved fibers, like microscopic bananas or staircases.
“We have a lot of control over the chemistry, the type of fibers, the architecture of the fibers, and how we deposit them,” Lahann says. “This really adds a lot of complexity to the way we can engineer surfaces now; not just with thin two-dimensional films but in three dimensions.”
Abbott is now a professor of chemical and biomolecular engineering at Cornell University. The Army Research Office supported the work.
Source: University of Michigan


