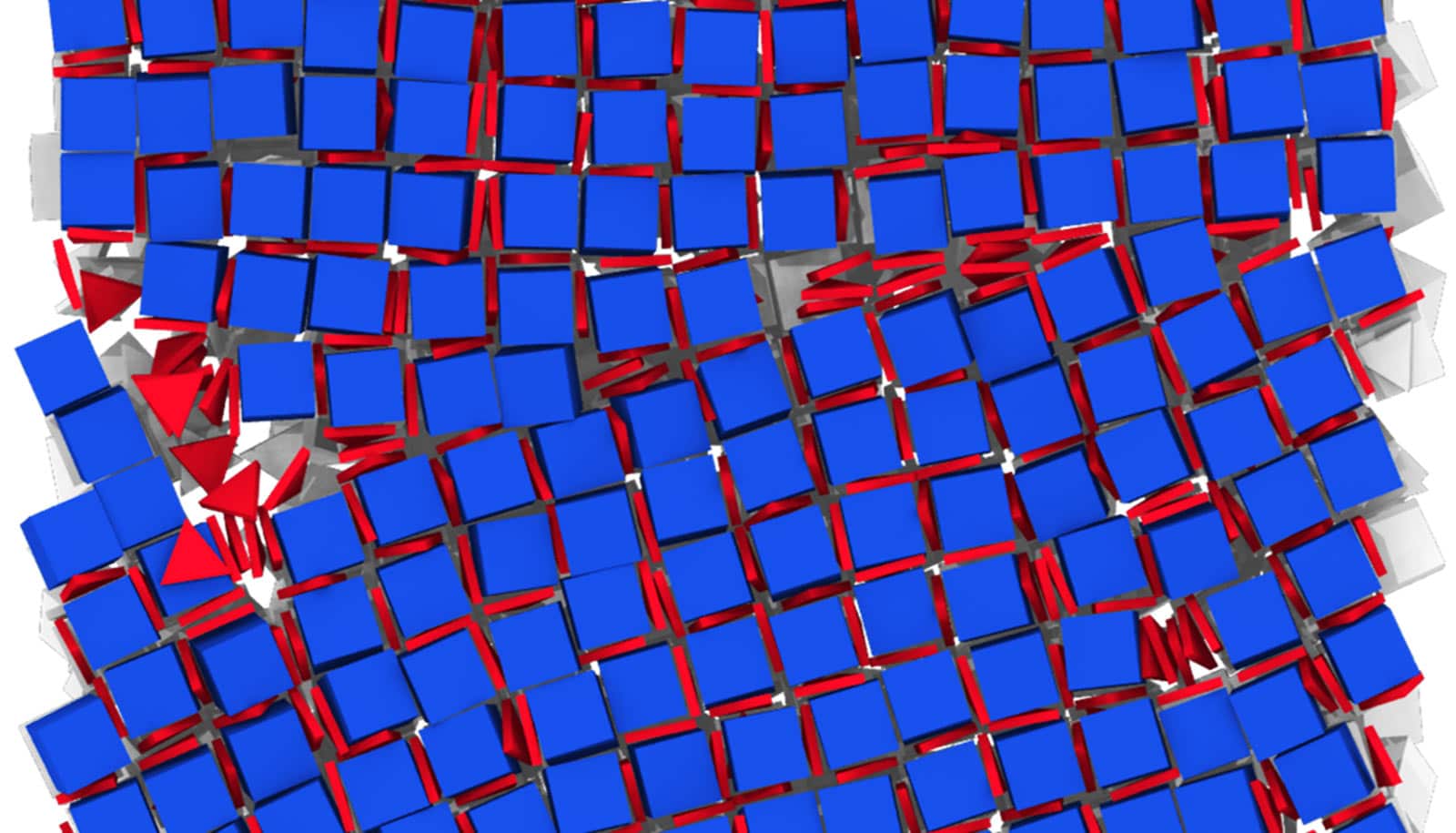
Researchers have developed a blueprint for designing new materials using difficult combinations of nanocrystals.
The work could lead to improvements in nanocrystals already used in displays, medical imaging, and diagnostics, and enable new materials with previously impossible properties.
Researchers can make materials with new and interesting properties by bringing together nanocrystals of different compositions, sizes, and shapes. The challenge is doing that in an organized way. Now, the team has developed a strategy that explores the available nanoparticles and figures out how to stick them together.
“It’s one of those problems where ‘like likes like,'” says recent PhD graduate Katherine Elbert, who led this study while working in the lab of Chris Murray, professor in materials science and engineering at the University of Pennsylvania.
This tendency means that the different kinds of nanocrystals often separate themselves, forming disordered blobs rather than integrated, ordered solids.
“Here, we’re trying to overcome that barrier and make materials in which the nanocrystals are precisely coupled to their neighbors to hybridize their properties,” Elbert says.
Computer modeling by the group of Sharon Glotzer, professor of engineering at the University of Michigan, demonstrated a way to sidestep this barrier by coating the nanoparticles with molecules that alter its shape as far as neighboring nanoparticles are concerned.
“We can leverage those subtle changes to drive assembly as opposed to segregation,” says Thi Vo, research fellow in chemical engineering at the University of Michigan.
One of the greatest challenges in the area of research is the sheer number and types of nanocrystals—with massive libraries of nanocrystals with varying chemical formulas, sizes, and shapes.
“Putting each ‘brick’ exactly in the right place would be insurmountable,” Murray says. “But if you can find the rules by which nature wants to assemble nanocrystals, and you know how to optimize the conditions and the precise design of blocks, you now have that blueprint for making different classes of materials.”
Glotzer’s group combed through the library of particles that Murray’s group could make, modeling interactions between pairs of nanocrystals to see how they might assemble themselves into different desired structures. The computational study recommended sizes, shapes, material types, and chemical environments for follow-up experiments in the lab.
The researchers focused on two classes of nanocrystals with vastly different compositions, sizes, and structures in this study—one with interesting optical properties and the other with useful electrical properties. Usually, they don’t like to mix. But if they did, we could potentially combine them to make solar cells that can convert infrared light to electricity more efficiently, among other possibilities.
When the team precisely controlled the surface sizes and shapes of the nanocrystals with those coating molecules, so that the right combinations of crystals would attract one another, they were able to create integrated structures. These results can be applied to other types of materials with only minor adjustments.
“By building nanoscale components and organizing them under a universal set of conditions, we can get materials properties that don’t coexist or are exceedingly difficult to bring together. Now, we have a strategy to get the nanocrystals to couple and overlap,” Murray says.
This research, which appears in the journal Science Advances, had support from the Office of Naval Research, as well as the National Science Foundation, the Department of Defense, the Air Force Office of Scientific Research, and the Vagelos Institute for Energy Science and Technology.
Source: University of Michigan