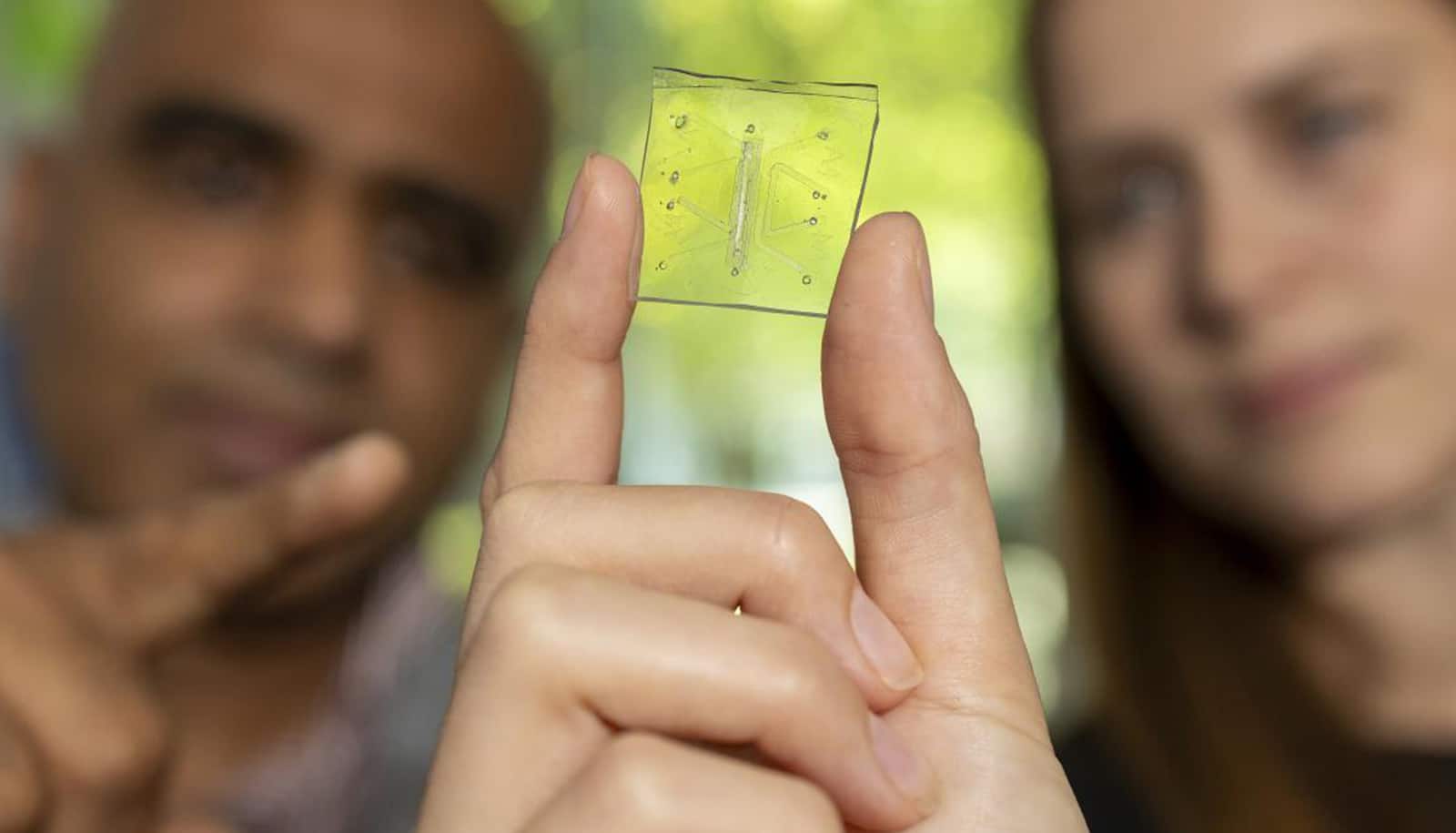
On a clear polymer chip, soft and pliable like a gummy bear, a microscopic lung comes alive—expanding, circulating, and, for the first time, protecting itself like a living organ.
For Ankur Singh, director of Georgia Tech’s Center for Immunoengineering, watching immune cells rush through the chip took his breath away.
Singh co-directed the study with longtime collaborator Krishnendu “Krish” Roy, former Regents Professor and director of the NSF Center for Cell Manufacturing Technologies at Tech and now the dean of engineering and University Distinguished Professor at Vanderbilt University.
“That was the ‘wow’ moment,” Singh says. “It was the first time we felt we had something close to a real human lung.”
Lung-on-a-chip platforms provide researchers a window into organ behavior. They are about the size of a postage stamp, etched with tiny channels and lined with living human cells. Roy and Singh’s innovation was adding a working immune system—the missing piece that turns a chip into a true model of how the lung fights disease.
Now, researchers can watch how lungs respond to threats, how inflammation spreads, and how healing begins.
For millions of people struggling with lung disease, everyday life can feel nearly impossible, whether it’s climbing stairs, carrying groceries, or even laughing too hard. Doctors and scientists have attempted for decades to unlock what really happens inside fragile lungs.
“This unique lung-on-a-chip model opens new, preclinical pathways of discovery that will allow researchers to better understand the interplay of immune responses to severe viral infections and evaluate critical antiviral treatments,” says Roy.
For Singh, a professor in the George W. Woodruff School of Mechanical Engineering with a joint appointment in the biomedical engineering department, this research is deeply personal. He lost an uncle when an infection overwhelmed his cancer-weakened immune system.
“That experience stays with you,” Singh says. “It made me want to build systems that could predict and prevent outcomes like that, so fewer families go through what mine did. I think about my uncle all the time. If work like this means fewer families lose someone they love, then it’s worth everything.”
That motivation pushed his team to reimagine what a lung-on-a-chip could do, setting the stage for the breakthroughs that followed.
The turning point came when Roy’s and Singh’s team peered through a microscope and saw something no one had ever witnessed on a chip: blood and immune cells coursing through tiny vessel-like structures, behaving just as they do in a living lung.
For years, researchers had struggled to add immunity to organ-on-a-chip systems. Immune cells often died quickly or failed to circulate and interact with tissue the way they do in people. the team solved that problem, creating a chip where immune cells could survive and coordinate a defense.
“It was an amazing breakthrough moment,” Singh says.
The true test came when the team introduced a severe influenza virus infection. The lung mounted an immune response that closely mirrored what doctors see in patients. Immune cells rushed to the site of infection, inflammation spread through tissue, and defenses activated in response.
“That was when we realized this wasn’t just a model,” Singh says. “It was capturing the real biology of disease.”
Singh and Roy’s research appears in the journal Nature Biomedical Engineering.
For decades, lung research has relied on animal models. But mice don’t get asthma like children. Their bodies don’t mount the same defenses.
“Five mice in a cage may respond the same way, but five humans won’t,” Singh explains. “Our chip can reflect that difference. That’s what makes it more accurate, and why it could dramatically reduce the need for animal models.”
Krish Roy emphasizes its potential.
“The Food and Drug Administration’s strategic vision on reducing animal testing and developing predictive non-animal models aligns perfectly with our work. This device goes further than ever before in modeling human severe influenza and providing unprecedented insights into the complex lung immune response,” he says.
What began with influenza now expands to a wider range of diseases. Roy and Singh believes the platform can be used to study asthma, cystic fibrosis, lung cancer, and tuberculosis. The researchers are also working to integrate immune organs, showing how the lung coordinates with the body’s defenses.
The long-term vision is personalized medicine: chips built from a patient’s own cells to predict which therapy will work best. Scaling, clinical validation, and regulatory approval will take years, but Singh is undeterred.
“Imagine knowing which treatment will help you before you ever take it,” Singh says. “That’s where we’re headed.”
Where we’re headed, the future doesn’t wait for illness. Instead, it anticipates it, intercepts it, and rewrites the outcome.
Support for this research came from Wellcome Leap, with additional funding from the National Institutes of Health, Carl Ring Family Endowment, and the Marcus Foundation.Rachel Ringquist, Roy’s graduate student, and now a postdoctoral fellow with Singh, led the work as part of her doctoral dissertation.
Source: Georgia Tech