Researchers have discovered a special antibody which may lead to a treatment for retinitis pigmentosa.
Retinitis pigmentosa (RP) causes loss of central vision, as well as night and color vision.
RP is a group of inherited eye diseases that affect the retina in the back of the eye. It is caused by the death of cells that detect light signals, known as photoreceptor cells. The condition has no known cure and development of new treatments relies on cell and gene therapies.
The researchers targeted their study on a specific molecule which they believe will provide a treatment for rhodopsin-associated autosomal dominant RP (adRP). The molecule, rhodopsin, is a key light-sensing molecule in the human retina. It is found in rod photoreceptor cells, and mutations in the rhodopsin gene are a primary cause of adRP.
“More than 150 mutations in rhodopsin can cause retinitis pigmentosa, making it challenging to develop targeted gene therapies,” says Krzysztof Palczewski, professor in the school of medicine at the University of California, Irvine. “However due to the high prevalence of RP, there has been significant investment in research and development efforts to find novel treatments.”
Although researchers have studied rhodopsin for over a century, key details of its mechanism for converting light into a cellular signal have been difficult to experimentally address.
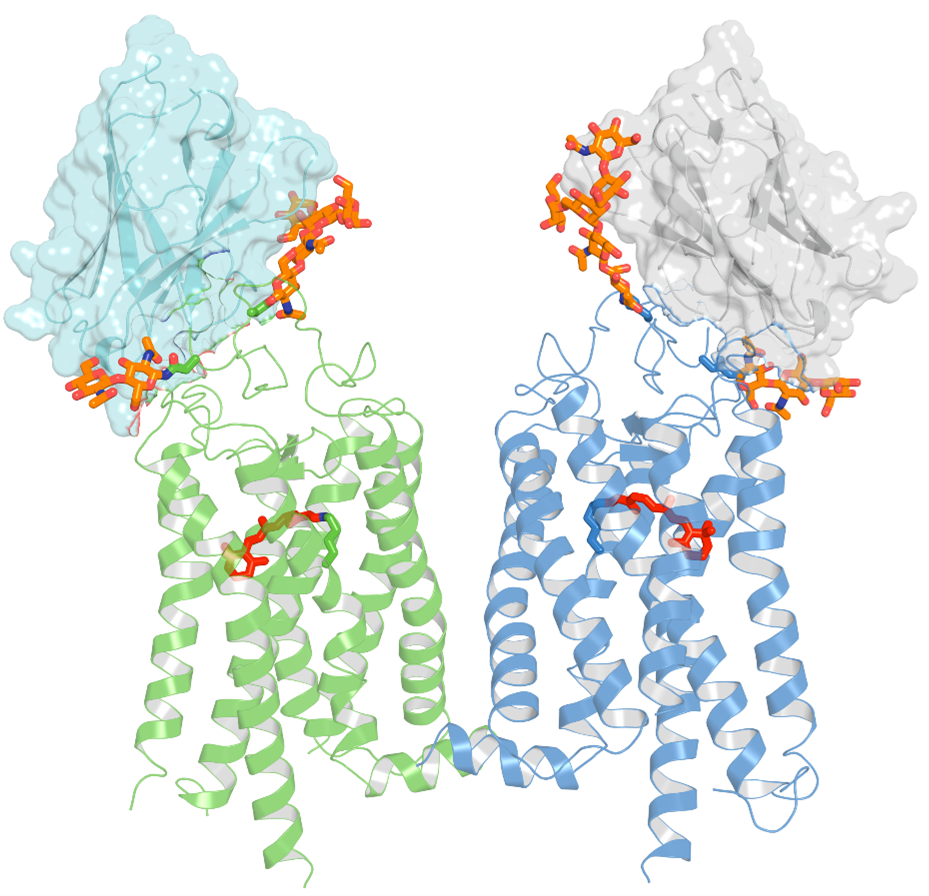
For this study, researchers used a special type of llama-derived antibody, known as a nanobody, that can halt the process of rhodopsin photoactivation, allowing it to be investigated at high resolution.
“Our team has developed nanobodies that work through a novel mechanism of action. These nanobodies have high specificity and can recognize the target rhodopsin extracellularly,” says David Salom, a researcher and project scientist at UCI School of Medicine. “This enables us to lock this GPCR in a non-signaling state.”
Scientists discovered that these nanobodies target an unexpected site on the rhodopsin molecule, near the location where retinaldehyde binds. They also found that the stabilizing effect of these nanobodies can also be applied to rhodopsin mutants that are associated with retinal disease, suggesting their use as therapeutics.
“In the future, we hope to involve the in vitro evolution of these initial set of nanobodies,” says Arum Wu, a researcher and project scientist, UCI School of Medicine. “We will also evaluate the safety and effectiveness of a future nanobody gene therapy for RP.”
The researchers hope to improve the nanobodies’ ability to recognize rhodopsin from other species including mice, for which several pre-clinical models of adRP are available.
They also have plans to use these nanobodies to address a long-term goal in the field of structurally resolving the key intermediate states of rhodopsin from the inactive state to the fully ligand-activated state.
The study appears in Nature Communications. This research received support from the National Eye Institute, the Department of Veterans Affairs, and the National Science Foundation.
Source: UC Irvine