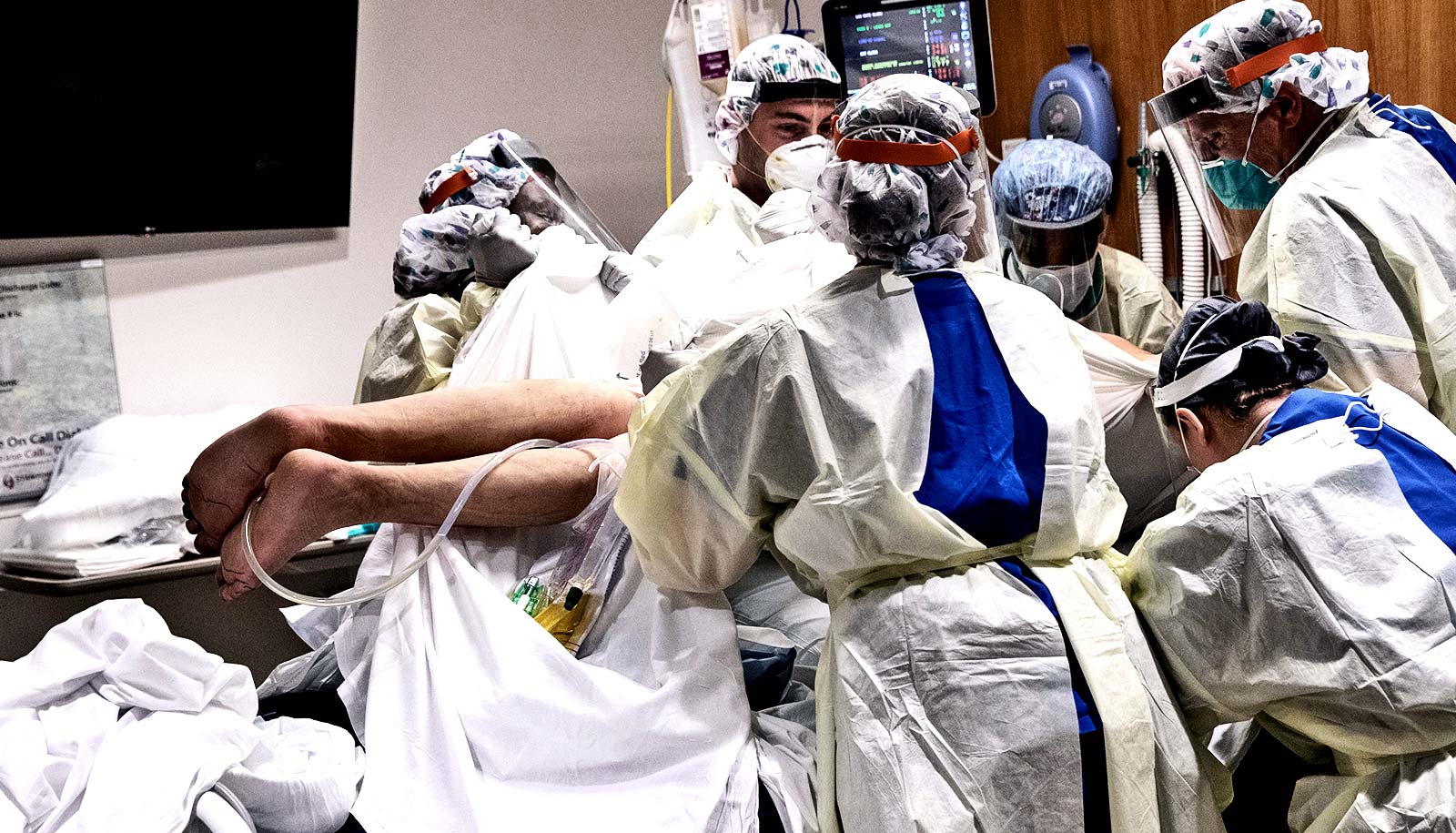
Flipping over may help patients with COVID-19 breathe more easily.
That could keep more patients from needing the ICU’s limited space and ventilators.
“It’s as simple as flipping on your stomach,” says Nicholas Bosch, a pulmonary and critical care fellow at Boston Medical Center (BMC) and a graduate researcher in epidemiology at Boston University’s School of Public Health.
One in four patients who arrive at BMC with COVID-19 go into the intensive care unit, says Bosch.
Bosch is leading a randomized controlled trial at BMC to see if having COVID-19 patients start lying prone (that is, on their stomachs) soon after arriving at the hospital can help keep their symptoms from getting worse. The trial will begin as soon as possible, pending final regulatory approval.
The study team includes over 50 students, faculty, staff, doctors, nurses, and department heads from BMC and Boston University’s Schools of Medicine and Public Health. “From top to bottom, there’s been huge interest and so many volunteers, and I don’t think the study would work without that,” Bosch says. “There would be no time, without that.”
Years before the new coronavirus emerged, research showed that prone positioning reduced deaths among patients with acute respiratory distress syndrome—the condition that is now often the cause of death in COVID-19 patients.
The idea, Bosch explains, is that the part of the lungs that is best at pulling oxygen into the blood is along a person’s back. When a patient lies on their back, that part of the lungs gets too much blood and not enough oxygen. Prone positioning gives that back part of the lungs a better ratio.
“It’s just gravity,” Bosch says.
In the current pandemic, many hospitals are now “proning” patients who already have severe COVID-19, including those on ventilators, and it seems to be helping.
“So much of what clinicians are doing with COVID right now is investigational, experimental,” but not in scientifically rigorous ways, Bosch says. As a low-tech and easily achieved clinical practice, proning seems worth studying properly, he says.
However, running a trial in a hospital right now is a major challenge, especially because there isn’t enough personal protective equipment (PPE) to spare for researchers.
That’s where Craig Ross comes in. Ross, a School of Public Health research assistant professor of epidemiology, created a smartphone-based system to gather data directly from patients remotely.
The system includes directions with pictures for patients to flip onto their stomach (careful not to pull out all the wires and tubes they’re routinely connected to), and reminds them to do it three times a day for an hour and at night.
“A couple times a day, Craig is also pinging them to say, ‘Will you fill out this survey?'” Bosch says. These surveys ask patients whether they are proning, for how long, and whether they are having any issues.
COVID-19 research also needs to move quickly, so that clinicians can start using effective strategies as soon as possible. To help with this aspect, Gheorghe Doros, professor of biostatistics in the School of Public Health, is providing expertise in adaptive study design for the trial.
Adaptive study uses the data gathered in an ongoing trial to change certain aspects of the trial in predetermined ways, instead of needing a series of separate, iterative studies to test new possibilities and zero in on strong results.
It can also be more ethical, Doros says: “In this study, if we learn from accumulating data that there is some indication that one treatment is better than the other, we’re going to assign more patients to that particular treatment. And, if the indication is overwhelming towards one of the treatments, we can stop the trial and say, ‘We have the answer,’ rather than waiting until the end.”
Source: Boston University