A new kind of raspberry-shaped silica particle has a different way to stabilizing emulsions—mixtures such as salad dressing, in which oil and vinegar are whisked together into a uniform texture.
It’s useful to stabilize emulsions because, given enough time, the finely dispersed vinegar droplets will fuse together again and the liquids will separate out completely.

As early as in the beginning of the 1900s, the British chemists W. Ramsden and S. U. Pickering also demonstrated that emulsions could be stabilized using very fine solid particles, such as spherical silica particles (SiO2).
In this process, the particles spontaneously enter and bind to the interface between the two liquids. They form a sort of armor around the droplets and prevent their fusion, thus stabilizing the emulsion practically indefinitely.
However, until now, this required two types of particles: those with hydrophilic surfaces, i.e. mostly sitting in the water, that stabilize only oil-in-water emulsions and those with hydrophobic surfaces, i.e. mostly sitting in the oil, that stabilize only water-in-oil mixtures.
Now, this may no longer be necessary: Lucio Isa, professor of interfaces, soft matter, and assembly in ETH Zurich’s department of materials, and colleagues have roughened the surfaces of these tiny silica spheres, which measure one to six micrometers in diameter, by loading them with silica nanoparticles of a much smaller diameter.
As a result, these tiny balls take on the shape of raspberries. Michele Zanini, a doctoral student in Isa’s group, altered the surface roughness in a controlled way and created a whole collection of such particles.
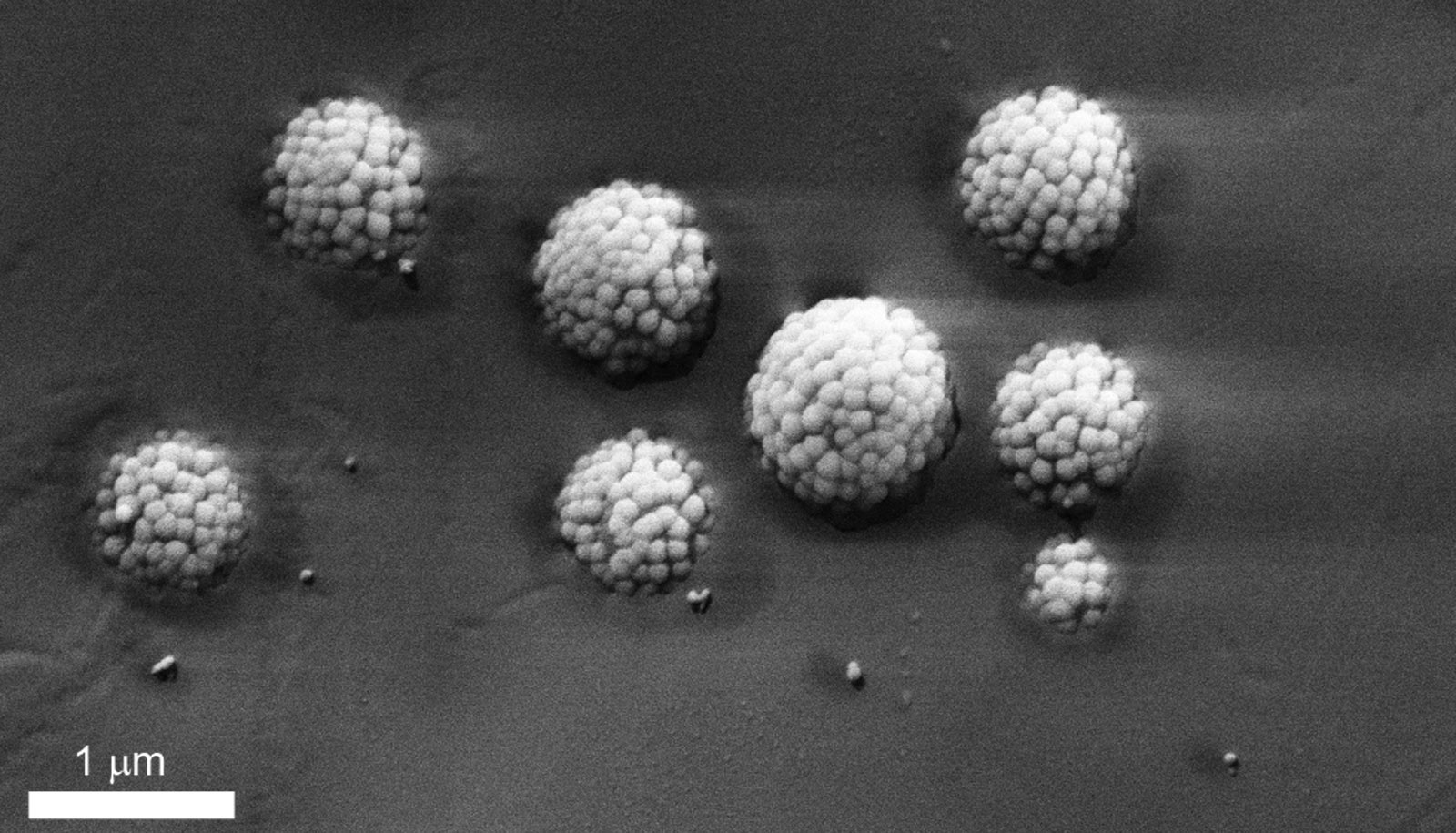
As reported in Nature Communications, the researchers have demonstrated that they can stabilize both types of emulsion using just one type of these raspberry-shaped particles. This depends solely on the liquid into which the particles are introduced before the emulsion is formed. If the researchers add the particles to the oil phase, a water-in-oil emulsion is formed. Conversely, they are able to stabilize an oil-in-water emulsion (oil droplets finely dispersed in water), if they dissolve their new particles in water first.
How droplets go from’ donut’ to sphere
“These particles can therefore be used as a universal tool for creating emulsions,” says Isa.
This is because the rough surface reduces the particles’ mobility through the droplet’s surface, he explains. “Although they push forward on the surface between the liquids, they cannot move as far across it as comparable silica particles with a smooth surface do—the rough particles get stuck before they can reach the energetically most favorable position at the interface,” says Isa.
With their raspberry-shaped particles, Isa and his colleagues have laid the foundation for further research in this area, and they have filed a patent for their new process of particle production as emulsion stabilizers.
There are many possible applications for these particles, such as in the chemical industry. Even though this research focused on laboratory model systems, the same principles can extend to the use of naturally occurring rough particles as emulsion stabilizers, to find other potential uses in the food, cosmetics, and pharmaceutical industries, though further research is needed in this direction.
Source: ETH Zurich



