Scientists have taken near-atomic resolution, 3D snapshots that show how a key biological machine works to fix potentially toxic, problematic proteins.
The machine is a protein complex called a disaggregase. It helps pull apart the threads of misfolded proteins that can accumulate and become toxic to cells—like the amyloid proteins associated with Alzheimer’s disease. The recovered proteins are then either refolded or destroyed to prevent dysfunction and maintain balance in the cell.
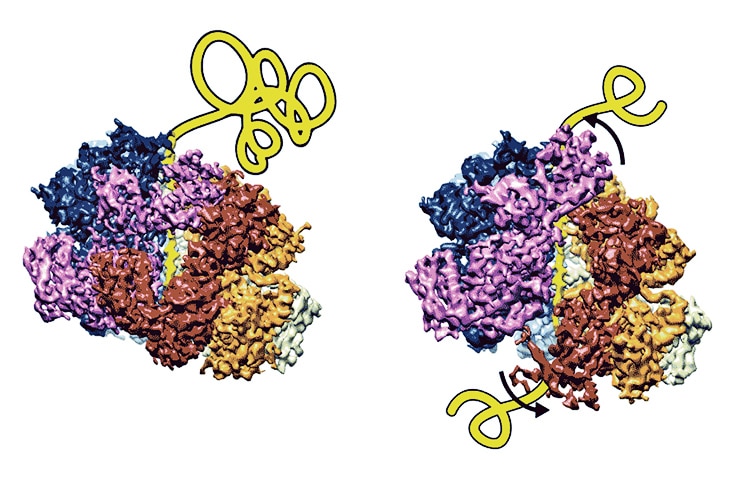
Scientists previously understood what the disaggregase did, but not precisely how it worked.
“It appears to pull substrates through stepwise, like a ratchet,” says senior study author Daniel Southworth, an assistant professor at the University of Michigan Life Sciences Institute, where his lab is located.
“It’s a very orderly process that moves around the machine’s six subunits. We can see how the proteins in the machine rearrange between different states to grab onto the next site on the substrate. There were several models that had been proposed for how this happens—and now, for the first time, we can start to see what’s actually occurring.”
Misfolded proteins get self-help nudge
The findings suggest there may be similar mechanisms at work more broadly across this important class of proteins, which are called AAA proteins—for ATPases Associated with diverse cellular Activities. Other members of the class, for example, are involved in DNA replication and repair. AAA proteins are found in plant and animal cells, as well as in bacteria and viruses.
A better understanding of cellular mechanisms can inform scientists’ work when they’re trying to develop new drugs or delve deeper into biological processes, Southworth says.
“Our study reveals how cells can break apart toxic protein aggregates to make them soluble and restore their function,” he says. “If we want to try to harness the power of these molecular machines, it’s important to have a clear picture of their mechanics.”
The structures were determined by using cryo-electron microscopy. Cryo-electron microscopy—or cryo-EM—is an evolving, cutting-edge imaging technology that involves instantaneously freezing proteins in a thin layer of solution. A focused beam of electrons is then used to reveal the shape of these very small, nanometer-sized objects. Specialized computer analysis is needed to combine hundreds of thousands of individual, two-dimensional snapshots in order to assemble the 3D shape at near-atomic resolution.
The technology can also sort out proteins that are in different stages of a biological process—thus helping to piece together how a biological machine moves, changes, and functions.
Possible rescue for misfolded proteins
The research took place in collaboration with researchers at the University of Pennsylvania. The findings appear in the journal Science.
Target ALS, the American Heart Association, the National Institutes of Health, the National Science Foundation, the Muscular Dystrophy Association, the Life Extension Foundation, and the Packard Center for ALS Research at Johns Hopkins University supported the research through grants and fellowship.
Source: University of Michigan



