A simple test aims to predict which treatment for cystic fibrosis is most likely to work for each patient, an approach called personalized, or precision, medicine, researchers report.
Several cutting-edge treatments have become available in recent years to correct the debilitating chronic lung congestion associated with cystic fibrosis. While the new drugs are life-changing for some patients, they do not work for everyone.
“Any given drug may not be the drug that works best for a given patient, because there’s so much variation from person to person,” says Jennifer Guimbellot, who conducted the research at University of North Carolina at Chapel Hill and is now assistant professor of pediatrics at the University of Alabama at Birmingham.
“We still have a long way to go to get a really optimized therapy for most cystic fibrosis patients, and the only way we can do that is to have a model like ours, where we can take cells from each individual patient and test them with each individual drug to find out which one is the best match,” Guimbellot says.
The right treatment for CF
From a drug-design perspective, cystic fibrosis is an enormous challenge. People develop CF because they do not have a functioning version of the CFTR gene. But the condition, which involves the buildup of thick, sticky mucus in the lungs, is tied to more than 2,000 genetic mutations. A person’s specific combination of mutations affects both the severity of the disease and how well the patient responds to treatment.
Recent years have brought hope in the form of several drugs known as CFTR modulators that counteract the effects of mutations in certain cystic fibrosis-linked genes.
Rather than just treating the symptoms, the drugs get to the root cause of cystic fibrosis by helping the body’s cells maintain the proper level of hydration. This, in turn, allows mucus to move freely in the lungs and other organs, preventing the buildup of sticky mucus that leaves cystic fibrosis patients prone to infections and other complications.
A trial-and-error approach for each patient—simply giving each available drug to every patient—is not feasible because the drugs cost hundreds of thousands of dollars, can take several months to work, and come with a risk of side effects. Currently, the only test available to predict how a patient will respond to CFTR drugs requires a rectal biopsy, followed by complicated laboratory manipulation of the sample to determine the drug’s likely effectiveness.
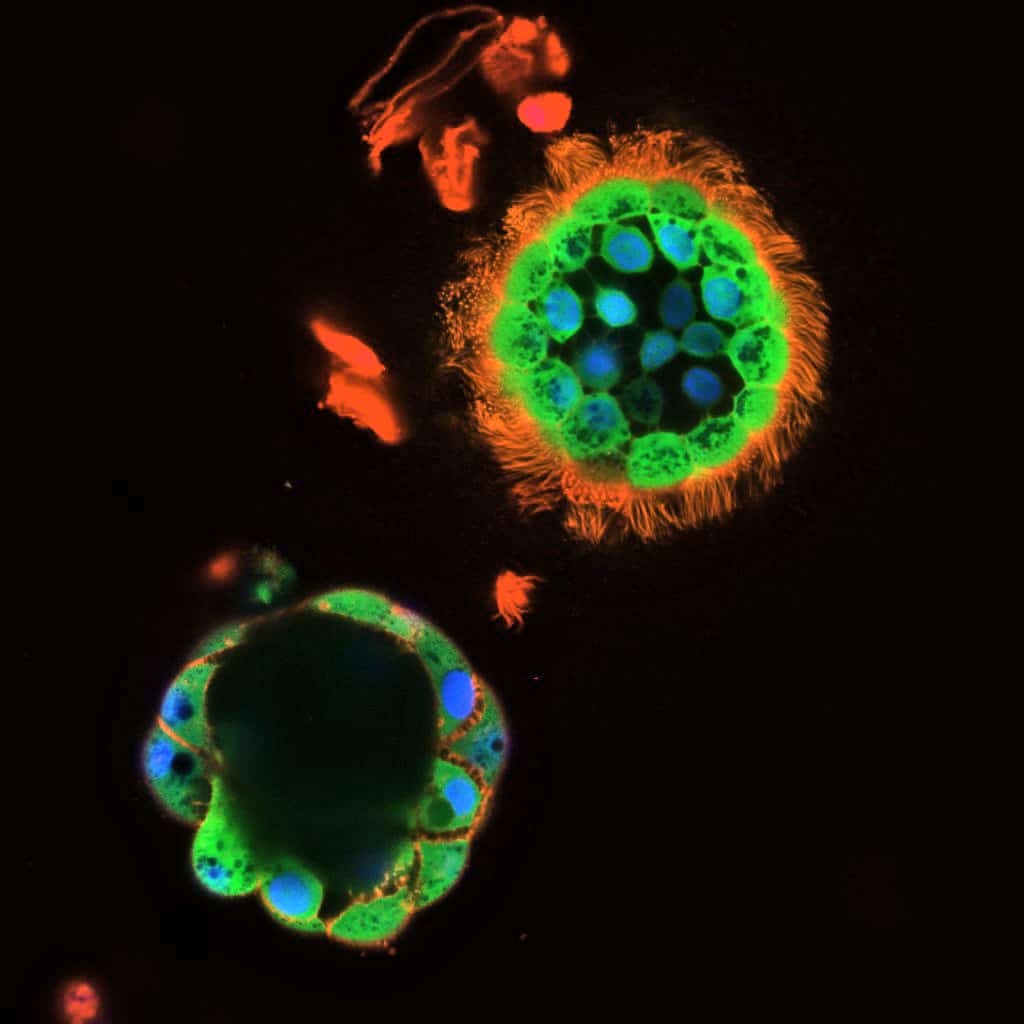
Guimbellot found another approach. She was working with lead study author Martina Gentzsch, associate professor of cell biology and physiology at the University of North Carolina, on an unrelated research project when she noticed some funny little balls forming from cells taken from the inside of the nose. The two scientists soon realized that the balls, which are called nasospheroids, were filled with fluid.
This gave them an idea. Maybe these nasospheroids could help to study essential parts of the CF hydration problem.
Simple and cheap
Fluid is at the heart of cystic fibrosis, since hydration of nasal and lung surfaces is what determines whether mucus is slippery or sticky. Guimbellot and Gentzsch learned how to grow nasospheroids from cells taken from the inside of the noses of cystic fibrosis patients. The researchers then exposed the nasospheroids to various CFTR drugs to see what would happen.
Enzyme may cause runaway inflammation in cystic fibrosis
“When CFTR is turned on, the balls shrink down—mediated by the transport of salt and water—and we can quantify that by measuring the size of the balls,” says Guimbellot.
The results suggest growing nasospheroids from nasal samples could provide a quick screening method to determine how a patient’s cells react to different CFTR drugs.
“It is a relatively simple procedure that doesn’t require any anesthesia and uses a brush that costs a few dollars,” says Gentzsch, a member of the UNC Marsico Lung Institute. “The spheroids form quickly in just a few days without much manipulation.”
Gentzsch says the testing approach could offer patients a welcome alternative to rectal biopsy. It might even be more reliable from a screening standpoint because the drugs are able to interact directly with the right parts on the outside of the nasospheroids, rather than having to enter the spheroids as required with the rectal biopsy method.
“It has many advantages, not only because patients should be able to get results really quickly, but also because our model is much more accessible to drugs for testing than the other existing models,” says Gentzsch.
The results represent a proof of concept, and the researchers note there is still much more work to do to validate the approach for clinical application.
“We have to establish how well this model can discriminate between small changes, and then do studies to see if it will predict for individual patients what their actual clinical outcomes would be,” says Guimbellot.
Cystic fibrosis sensor gives salt a glow
Researchers report their findings in the Journal of Clinical Investigation Insight.
The North Carolina Translational and Clinical Sciences (NC TraCS) Institutel the Cystic Fibrosis Foundation; a Kaul Pediatric Research Institute Pilot Award; and the National Institutes of Health funded this work.
Guimbellot and Gentzsch are inventors of the technology “airway sphere cultures as diagnostic device to monitor pharmacological responses of ion channels,” which is licensed to Path BioAnalytics. As inventors, they could receive royalties related to the license in the future. They have disclosed these relationships to and are under management by the University of North Carolina at Chapel Hill.
Source: UNC-Chapel Hill