Experimental PET scans on living people can detect abnormal brain tissue—called tau protein—in patterns similar to those in the brains of deceased people diagnosed with CTE after death, report researchers.
For the time being, the only way for scientists to detect whether a person has CTE, or chronic traumatic encephalopathy, is to examine their brain tissue after death. But to get any closer to being able to treat or even prevent CTE, researchers must first find a way to diagnose it in the living. That critical goal may finally be within sight, according to a new study, which appears in the New England Journal of Medicine.
Tau protein—a hallmark of several neurodegenerative diseases including CTE, Alzheimer’s disease, and certain kinds of dementias—”becomes toxic and destroys brain tissue” as it accumulates, says Robert Stern, director of clinical research at the Boston University Chronic Traumatic Encephalopathy (CTE) Center.
In their study, the researchers found evidence of abnormal tau proteins in living people when they compared experimental PET (positron emission tomography) scans of 31 control subjects without any history of head trauma or psychological symptoms against 26 former National Football League players who have self-reported cognitive, mood, and behavior symptoms associated with CTE.
The experimental PET scan detected greater amounts of abnormal tau protein buildup in the group of living former NFL players compared to the control group.
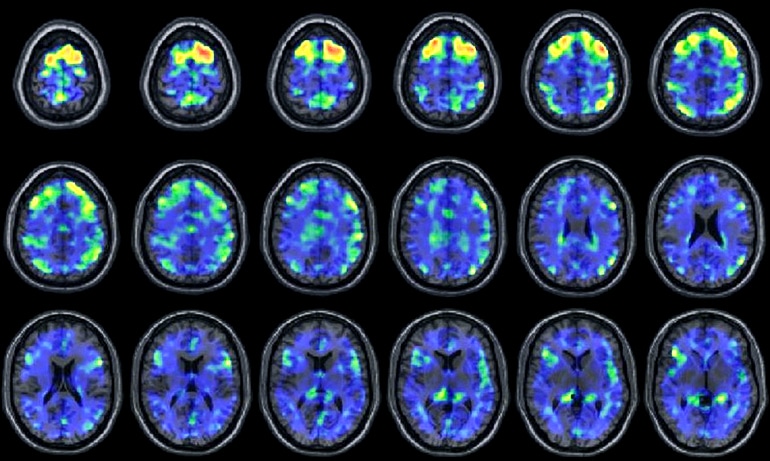
“It can’t yet be used for individual diagnosis,” Stern cautions. “We analyzed group data, not individual findings.”
Yet, after a decade of doing CTE research himself, he admits it’s a crucial step toward the ultimate goal of diagnosing CTE in living individuals.
“From day one,” Stern says, “I had hoped for there to be a tau tracer for PET scans in humans. It’s such an important thing… to be able to see and quantify tau.”
Searching for answers
Stern says scientific data suggests that it’s not necessarily concussions that cause the neurodegenerative disease known to have affected hundreds of military veterans and former NFL stars, including Aaron Hernandez, Dave Duerson, Junior Seau, and Andre Waters, among others.
“CTE is a buzzword these days,” Stern says. But “a lot of people are confused about what it is, what causes it…. There’s a lot of misconception out there that it’s caused by concussions.”
Instead, repetitive subconcussive hits to the head—like you commonly see in tackle football—appear to be the root of the disease. Studying the brains of deceased NFL players, other athletes, and veterans—scientists have amassed nearly 700 such brains—has yielded new clues to what drives CTE. But Stern says there are still a lot of unanswered questions, such as how common CTE is, why some people get it and others don’t, and how to treat and possibly prevent it.
To find those answers, he says researchers need to be able to diagnose CTE in the living.
“Most importantly,” to best learn about how and why the disease develops, “it would be great to detect it early before it progresses to the point where there’s too much destruction of brain tissue,” says Stern, who is also director of the clinical core of the Boston University Alzheimer’s Disease Center and a professor of neurology, neurosurgery, and anatomy, and neurobiology in the School of Medicine.
Tracing tau
The researchers performed the experimental PET scans in the new study using two different tracers—radioactive compounds designed to be injected into the bloodstream, after which they travel into the brain and glom onto specific proteins.
Researchers looking for signs of Alzheimer’s disease have used the two types of tracers Stern’s team used, one an experimental tracer designed to detect tau and the other an FDA-approved tracer for detecting amyloid proteins, over the past several years.
Once these tracers, delivered one at a time during two different PET scans, reach the brain and get stuck to any existing tau and amyloid proteins, the PET scans can pick up their radioactive glow, illuminating their exact location and pattern inside the brain structure.
The FDA-approved amyloid tracer is intended “to be used in people in their 60s and above who have cognitive difficulties, but their doctor isn’t sure if it’s Alzheimer’s,” Stern says. “If they have the PET scan and it comes back negative [without any elevated amyloid], the doctor can’t assume the person has Alzheimer’s.”
Because of tau’s role in Alzheimer’s—which affects more than 5.5 million Americans and is now the sixth leading cause of death in the United States, according to the National Institute on Aging—researchers raced to develop tau tracers for use with PET scans. Stern says the combination of the two scans, amyloid and tau, may help detect the specific brain tissue patterns that make CTE unique from other neurodegenerative diseases.
“At the beginning of CTE, tau is found in patchy areas around small blood vessels located deep in the valleys of the cortex,” he says. From there it can spread throughout other areas of the brain, until the whole brain can become “devastated.”
In contrast, Alzheimer’s disease starts off “almost backward from CTE development,” Stern says. Deep in the brain, “amyloid buildup seems to kick off first and then tau becomes abnormal,” forming protein tangles along the spreading amyloid network.
In CTE, then, you would expect to find a smattering of tau protein without the presence of elevated amyloid.
The results from the experimental PET scans in the study seem consistent with those facts. Although researchers used both tau and amyloid tracers in the study, they only detected abnormal tau in the group of former NFL players. As expected with CTE, there were no abnormal signs of amyloid buildup.
1 step in a long journey
Looking at the group results as a whole, Stern says there’s no way to interpret whether any individual person in the study has CTE, but he says, “it’s likely that there are people in the group who have CTE.”
Ultimately, these early results are a step—albeit a significant one—in the journey toward one day being able to diagnose individuals with CTE while they are still alive.
“We need to study larger numbers of people with greater variability in their history of being hit [in the head] repeatedly and in their history of CTE-related symptoms,” he says. By the end of 2019, Stern and collaborators expect to complete tau and amyloid scans of up to 240 additional people. “In the next five years or so, we will be able to diagnose and detect [CTE] during life.”
But whether or not the experimental PET scans on this group detected signs of CTE, or some other abnormality, won’t become clear unless researchers examine the study participants’ brains after death. To make that possible someday, Stern says, “most of them have agreed to donate their brains.”
Additional researchers from Avid Radiopharmaceuticals, Banner Alzheimer’s Institute, Brigham and Women’s Hospital, the Mayo Clinic, and University of Arizona contributed to the research. Avid Radiopharmaceuticals, the National Institutes of Health, and the Department of Defense supported the work.
Source: Boston University



