Researchers have developed a way to protect against corrosion after a chance discovery.
The material glows in places where it is not damaged, repairs itself, and can be reused multiple times.
Skyscrapers, bridges, ships, airplanes, cars—everything humans make or build sooner or later decays. The ravages of time are known as corrosion; nothing is safe from it.
This makes the fight against corrosion expensive. All countries together invest around 3.5% of annual global gross domestic product in corrosion protection, which amounts to some $4,000 billion: a huge market—and a gigantic problem.
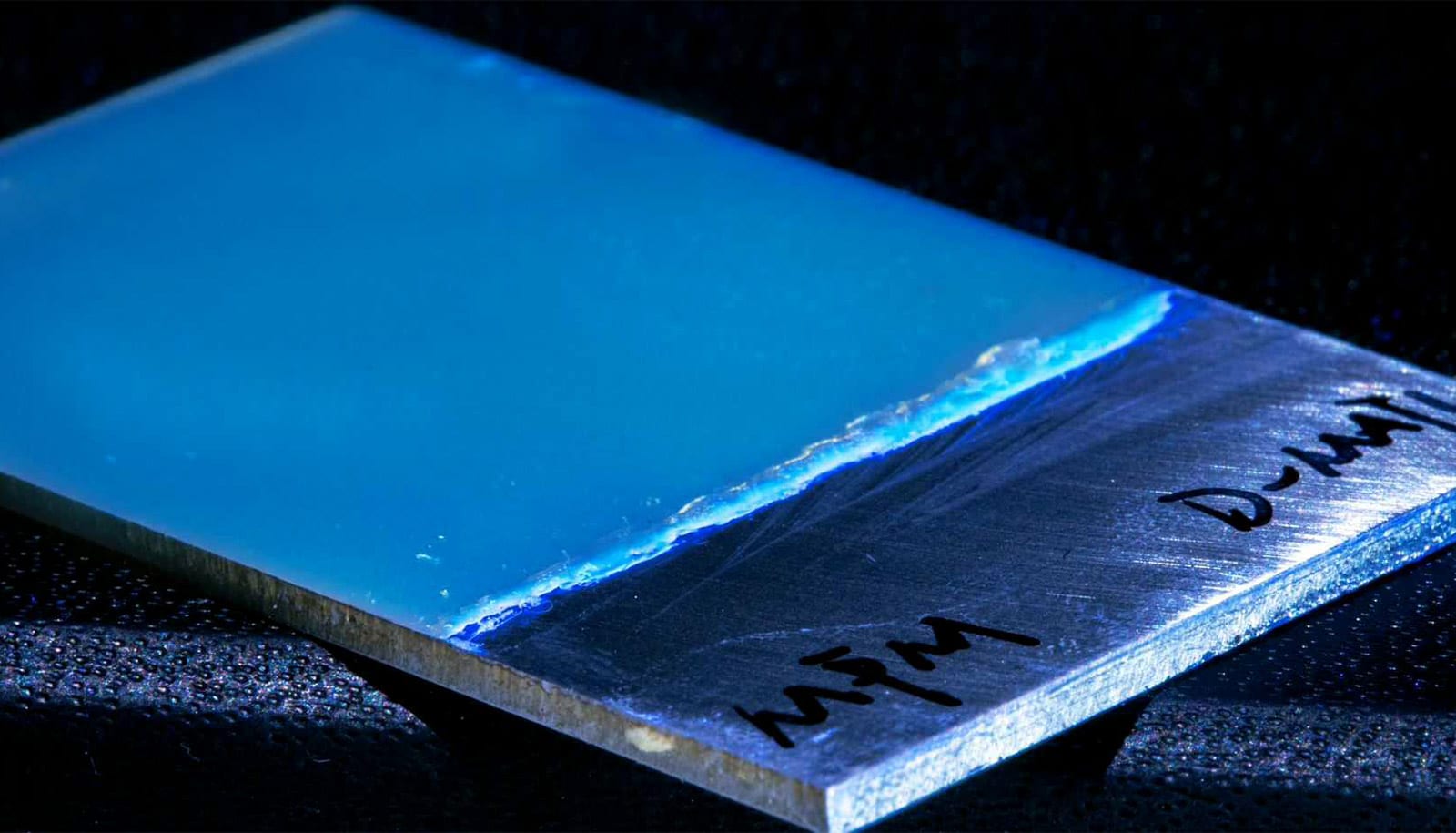
The new corrosion protection material, called poly(phenylene methylene), or PPM for short, kills several birds with one stone. When mixed as paint and heated, PPM can be sprayed onto a surface and becomes solid. The polymer indicates holes and cracks in the protective layer by failing to fluoresce.
What’s more, it repairs any damage itself without further external intervention. And at the end of a product’s life, the polymer can be completely removed and recycled with only minimal material loss. The recycled polymer can then be applied to another surface with no loss in its special properties and functions.
Surprise fluorescence
It was pure chance that kicked off this development. About 10 years ago, Markus Niederberger and Walter Caseri from the Laboratory for Multifunctional Materials at ETH Zurich, were working on the production of nanoparticles in a special organic solvent. Under certain conditions, the solvent became solid: it polymerized.
“That was unintentional and unwanted,” Niederberger recalls. “We didn’t know what to do with it at first either.”
But then they discovered that the polymer they had created by accident—known as PPM—had another interesting property in addition to its high thermal stability: it fluoresced even though conventional knowledge suggested it should not be fluorescent at all. And so the researchers specifically refined the material. First, a doctoral student improved the polymer’s synthesis. After that, his successor, the doctoral student Marco D’Elia, was given the task of finding a useful application for PPM.
“And he did this job with flying colors,” says Caseri, who supervised D’Elia. His contacts with corrosion experts at the Università degli Studi di Milano also proved fruitful, Caseri says.
Recyclable corrosion protection
Laboratory tests revealed that a PPM-based coating protects metals, especially aluminum, well against corrosion. Even though this protective coating can be applied in layers that are up to 10 times thinner than conventional protective agents—such as those based on epoxy resins—it is durable.
Last but not least, the polymer seals any damage to the coating by itself.
“Self-repair mechanisms are in great demand, but they’re very difficult to attain, and good solutions are still rare,” Caseri says. To achieve self-repair usually requires chemical additives, which migrate in the polymer over time and are released into the environment. Not so with PPM: “This material doesn’t require any additives,” Caseri says.
PPM is also more sustainable than previous corrosion protection materials because it can be completely removed and recycled at the end of the product’s life. While some polymer material is lost in the process, the recycling rate is very high at 95%. In their tests, the researchers were able to reuse the material five times.
Studies on the sustainability of PPM-based corrosion protection also show that the polymer performs better than epoxy-based corrosion protection materials when it comes to both environmental impact and human health.
“There are really only two disposal solutions for epoxy resins: incineration or landfill,” D’Elia says. “Our product allows for a third solution: recycling.”
Improvements needed
Nevertheless, PPM corrosion protection is not completely harmless to the environment.
“Synthetic products always have an impact,” D’Elia says. “But if you choose the right approach, you can limit that impact to a great extent.” The former doctoral student hopes to see PPM corrosion protection commercialized.
The researchers have applied for a patent for their invention. It is still pending. They are also currently looking for an industry partner to further develop the product and to manufacture and distribute it on a large scale. Given the size of the global market, D’Elia sees huge potential for PPM.
“Our technology is pretty advanced, but before we can sell it as a product, there are still some improvements for us to make,” he says.
Caseri, in turn, is proud of what has been achieved. The chemical synthesis, the characterization of PPM’s molecular structure and the study of material properties that were not expected for this type of polymer—such as fluorescence —show “all the versatility of materials science.”
In addition, manufacturing, another mainstay of his department, also got a chance to shine, he says. “And now we have a great application. We’ve covered all these fundamental elements of materials science with this project.”
The study appears in the journal Polymers.
Source: ETH Zurich