Researchers have determined how much human-made CO2 emissions the ocean took up between 1994 and 2007.
Not all of the CO2 that the combustion of fossil fuels generates remains in the atmosphere and contributes to global warming. The ocean and the ecosystems on land take up considerable quantities of these human-made CO2 emissions from the atmosphere.
The ocean takes up CO2 in two steps: first, the CO2 dissolves in the surface water. Afterwards, the ocean’s overturning circulation distributes it: ocean currents and mixing processes transport the dissolved CO2 from the surface deep into the ocean’s interior, where it accumulates over time.
This overturning circulation is the driving force behind the oceanic sink for CO2. The size of this sink is very important for the atmospheric CO2 levels: without this sink, the concentration of CO2 in our atmosphere and the extent of anthropogenic climate change would be considerably higher. Determining what share of the human-made CO2 the oceans absorb has long been a priority for climate researchers.
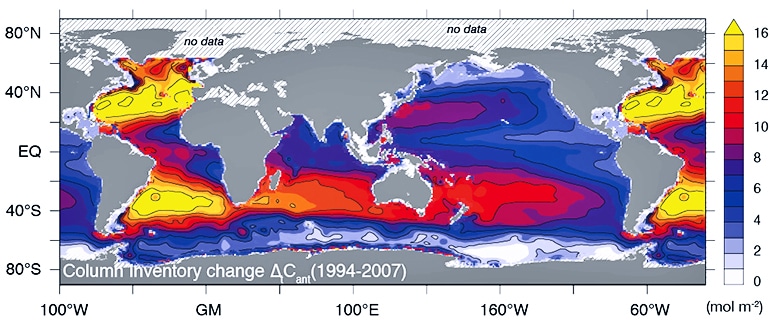
As reported in Science, the researchers found that the ocean took up as much as 34 gigatonnes (billions of metric tons) of human-made carbon from the atmosphere between 1994 and 2007. This figure corresponds to 31 percent of all anthropogenic CO2 emitted during that time.
The percentage of CO2 the oceans take up has remained relatively stable compared to the preceding 200 years, but the absolute quantity has increased substantially. This is because as long as the atmospheric concentration of CO2 rises, the oceanic sink strengthens more or less proportionally: the more CO2 is in the atmosphere, the more the oceans absorb—until they become eventually saturated.
So far, that point has not been reached. “Over the examined period, the global ocean continued to take up anthropogenic CO2 at a rate that is congruent with the increase of atmospheric CO2,” says Nicolas Gruber, professor for environmental physics at ETH Zurich.
The sink still works
These data-based research findings also confirm various earlier, model-based estimates of the ocean sink for human-made CO2.
“This is an important insight, giving us confidence that our approaches have been correct,” Gruber adds. The results further allow the researchers to draw conclusions about the CO2 sink of the ecosystems on land, which are more difficult to determine.
While the overall results suggest an intact ocean sink for human-made CO2, the researchers also discovered in the different ocean basins considerable deviations from the uptake expected from the rise in atmospheric CO2. The North Atlantic Ocean, for instance, absorbed 20 percent less CO2 than researchers expected between 1994 and 2007.
“This is probably due to the slowdown of the North Atlantic Meridional Overturning Circulation in the late 1990s, which itself is most likely a consequence of climate variability,” Gruber explains. But a considerably greater uptake in the South Atlantic offset this lower sink in the North Atlantic, such that the entire Atlantic’s uptake developed as expected.
The researchers documented similar fluctuations in the Southern Ocean, the Pacific, and the Indian Ocean.
“We learned that the marine sink does not just respond to the increase in atmospheric CO2. Its substantial sensitivity to climate variations suggests a significant potential for feedbacks with the ongoing change in climate,” Gruber emphasizes.
Before and after industrialization
The researchers based their results on a global survey of CO2 and other chemical and physical properties in the various oceans, measured from the surface down to depths of up to 6 kilometers (about 3.72 miles). Scientists from seven countries participated in the internationally coordinated program that started in 2003. Globally, they carried out more than 50 research cruises through 2013, when they synthesized their findings into a global data product.
For their analyses, the researchers used a new statistical method Gruber and his former PhD student Dominic Clement developed. This method allowed them to distinguish between the changes in the human-made and the natural CO2 components that make up the changes in the total concentration of dissolved CO2 in the water. Natural CO2 refers to the amount of CO2 that existed in the oceans prior to industrialization.
Gruber had already participated in a similar study around the turn of the millennium. Using observations researchers obtained from the very first global CO2 survey conducted between the late 1980s and the mid-1990s, that study estimated that the ocean had taken up around 118 gigatonnes of carbon from the beginning of industrialization around 1800 until 1994. His current team of researchers extended this analysis up to 2007, permitting them not only to establish the budget for human-made CO2 for the 1994 through 2007 period, but also to assess the intactness of the ocean carbon sink.
By moderating the rate of global warming, the oceanic sink for human-made CO2 provides an important service for humanity, but it has its price: the CO2 dissolved in the ocean acidifies the water.
“Our data has shown that this acidification reaches deep into the ocean’s interior, extending in part to depths of more than 3000 [meters (about 1.86 miles)],” Gruber says.
This can have serious consequences for many marine organisms. Calcium carbonate spontaneously dissolves in acidified environments, which poses a hazard to mussels and corals whose shells and skeletons are made of calcium carbonate. The changing chemical composition of the ocean can also impact physiological processes such as the breathing of fish.
“Documenting the chemical changes imparted on the ocean as a result of human activity is crucial, not least to understand the impact of these changes on marine life,” Gruber says.
Source: Michael Keller for ETH Zurich



