Researchers have created nanoscale protein micelles capable of delivering chemotherapeutic drugs while scientists track it with magnetic resonance imaging (MRI).
The innovation falls into the category of “theranostics,” meaning that it combines diagnostic capability and drug delivery, allowing researchers to administer therapy while also non-invasively monitoring the therapeutic progress and drastically reducing the need for surgical intervention.
“Think of the analogy of a missile aimed at a target, with the chemotherapeutic drug as the missile and the cancer cells as the target. It’s not enough to aim blindly; you need to carefully track the missile’s progress and determine to what extent it is effective,” says Jin Kim Montclare, a professor of chemical and biomolecular engineering at the Tandon School of Engineering at New York University.
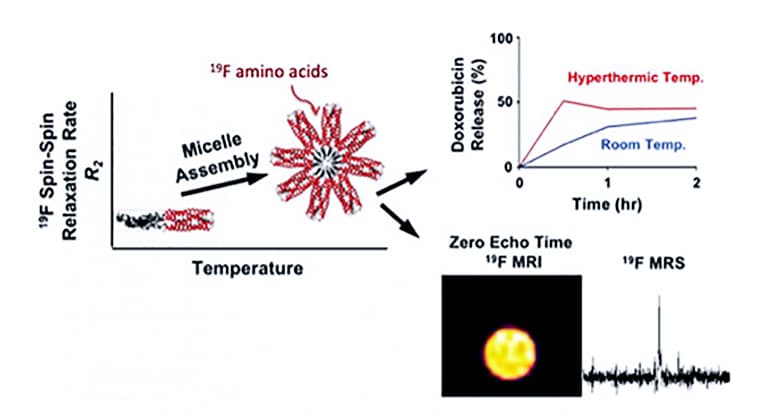
In a new paper in ACS Nano, the researchers explain that engineered proteins provide an interesting template for designing fluorine-19 (19F) MRI contrast agents, but the unpredictable relaxation properties of fluorine have hindered progress. MRI relies upon detecting differences in the relaxation rates of the protons of water molecules within tissue, but there are times when the rates do not differ sufficiently between tissue types to produce useful contrast.
As a solution, the researchers present the biosynthesis of a protein block copolymer containing amino acid building blocks with 19F, which they call “fluorinated thermoresponsive assembled protein” (F-TRAP), which assembles into a nanoscale micelle with noteworthy imaging properties along with the ability to encapsulate and release small therapeutic molecules.
Previously, Montclare had developed a protein-lipid system capable of carrying not only small-molecule therapeutic drugs but nucleic acids for gene therapy at the same time, as a dual payload, in order to treat cancer, diabetes, and other conditions requiring a variety of therapeutic approaches.
The National Science Foundation and the National Institutes of Health National Center for Advancing Translational Sciences funded the research.
Source: New York University



