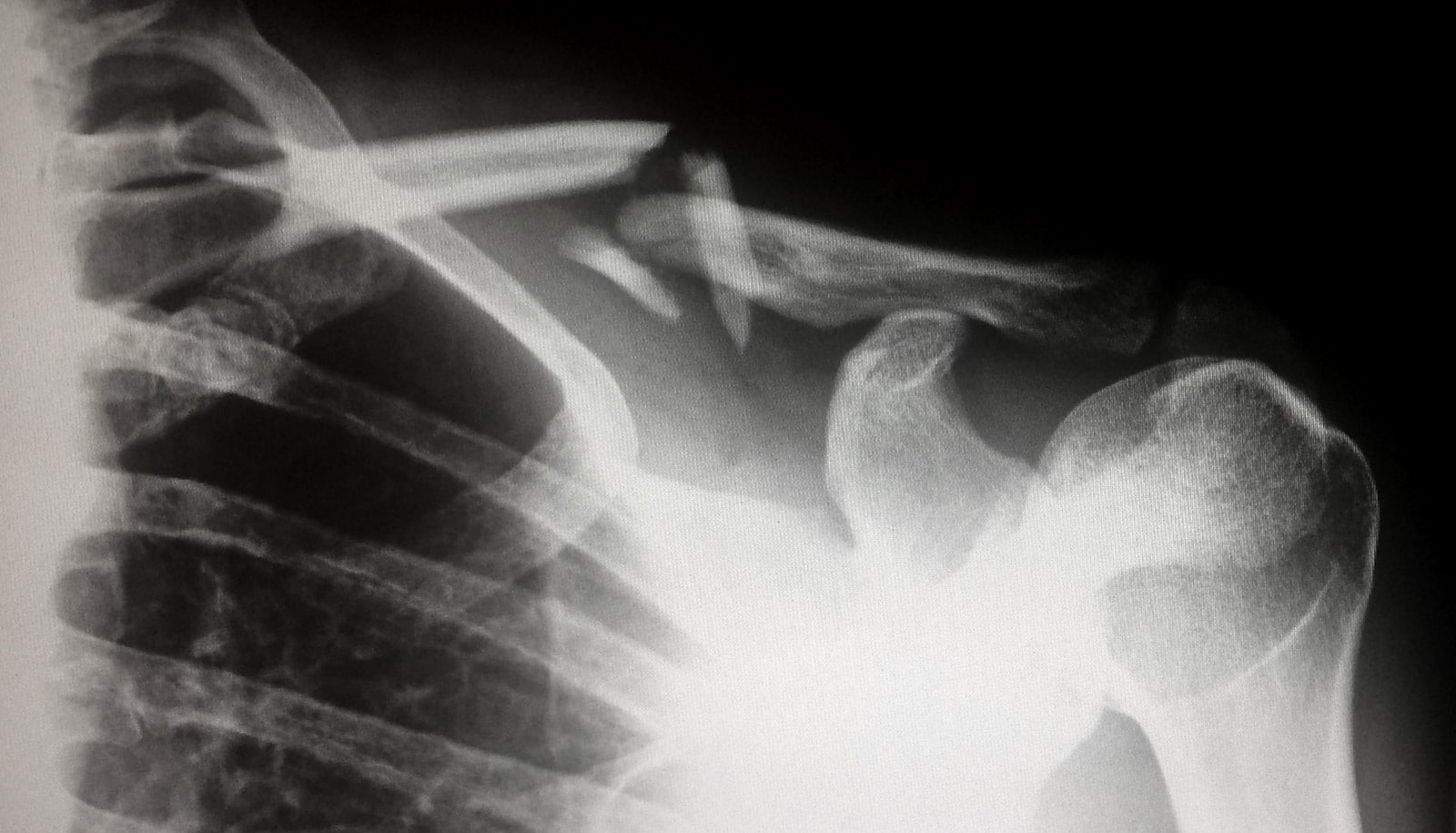
A radioactive bone cement that’s injected into bone to provide support and local irradiation is proving to be a safer alternative to conventional radiation therapy for bone tumors, according to a new study.
This brachytherapy cement placed into spinal bones directly irradiates tumors without harming the spinal cord. Further, the radioactive material will stay localized in the bones, which promises to virtually eliminate side effects.
Cancers that begin in the breast, prostate, lung, thyroid, kidney, and other locations can spread to and erode bones, most commonly in the spine. Further complicating matters, normal radiation treatments for this problem can threaten the spinal cord and weaken the bones that the tumor’s erosion have already compromised, increasing the risk of fracture.
Currently, doctors use multiple sessions of external beam radiation to treat cancer that has spread to the spine. This radiation causes unpleasant side effects, including nausea, vomiting, and diarrhea and passes through the spinal cord, which often delays and limits treatment.
“Brachytherapy cement could be used without delay in a convenient, one-step, minimally invasive treatment to irradiate tumors and would not irradiate the spinal cord or limit future treatment options,” says lead researcher Joyce Keyak, professor of radiological sciences at the University of California, Irvine.
In animal and computational studies, the researchers evaluated the short-term safety of injecting brachytherapy cement into vertebrae; the possible migration of radioactivity into blood, urine, or feces; the dose rate outside the injection site; and the radiation dose from phosphorus-32 emissions to the spinal cord and soft tissue.
At 17 weeks post-injection, physical examinations were all normal and researchers detected no activity in blood, urine, or feces. They found no evidence of the P-32 isotope in the circulating blood, no changes in blood work related to radioactivity, and no neurological deficits.
“This localized treatment for bone tumors stays localized, and we did not see any effects outside the bone,” Keyak says. “This is important because traditional radiation therapy causes adverse effects such as nausea, vomiting, and diarrhea.”
To create the brachytherapy cement, Keyak and Harry Skinner, an orthopedic surgeon with St. Jude Heritage Medical Group, infused a common product of their trade, bone cement, with radioactive material already used in other treatments.
The brachytherapy bone cement does not have the same side effects as traditional radiation therapy, Keyak says because the injection directly targets the tumor and radiation doesn’t pass through other organs, such as the intestines or stomach.
“You can have this procedure and be done with it.”
Previous studies also revealed that it can immediately reduce pain in the spine, potentially getting patients off strong painkillers that could carry additional side effects.
Normally, a bone cancer patient needs 10 or more sessions of radiation therapy. But with the brachytherapy bone cement, a single injection can provide an equivalent, targeted tumor treatment with significantly less threat to the spinal cord and nerves, Keyak says.
“You can have this procedure and be done with it,” she says. “And you can do it when tumors are smaller to prevent further bone and spinal cord damage, while limiting the pain and side effects that patients often feel.”
Keyak and Skinner have started a company, Bone-Rad Therapeutics, for their product and have licensed its intellectual property (four patents and one pending patent).
The next step will be more animal studies, followed by an application for a clinical trial, Keyak says. She presented her findings at the virtual 2021 annual meeting of the Orthopaedic Research Society.
Additional coauthors are from Pedicaris Research in Birmingham, Michigan and UC Irvine.
Source: UC Irvine