New research demonstrates how biological sex can modify communication between nerve cells and generate different responses to the same stimulus in males and females.
The findings, which appear in the journal Current Biology, could shed new light on the genetic underpinnings of sex differences in neural development, behavior, and susceptibility to diseases.
“While the nervous systems of males and females are virtually identical, we know that there is a sex bias in how many neurological diseases manifest themselves, that biological sex can influence behavior in animals, and that some of these differences are likely to be biologically driven,” says lead author Douglas Portman, an associate professor in the biomedical genetics and neuroscience departments and the Center for Neurotherapeutics Discovery at the University of Rochester Medical Center.
“This study demonstrates a connection between biological sex and the control and function of neural circuits and that these different sex-dependent configurations can modify behavior.”
What worms reveal
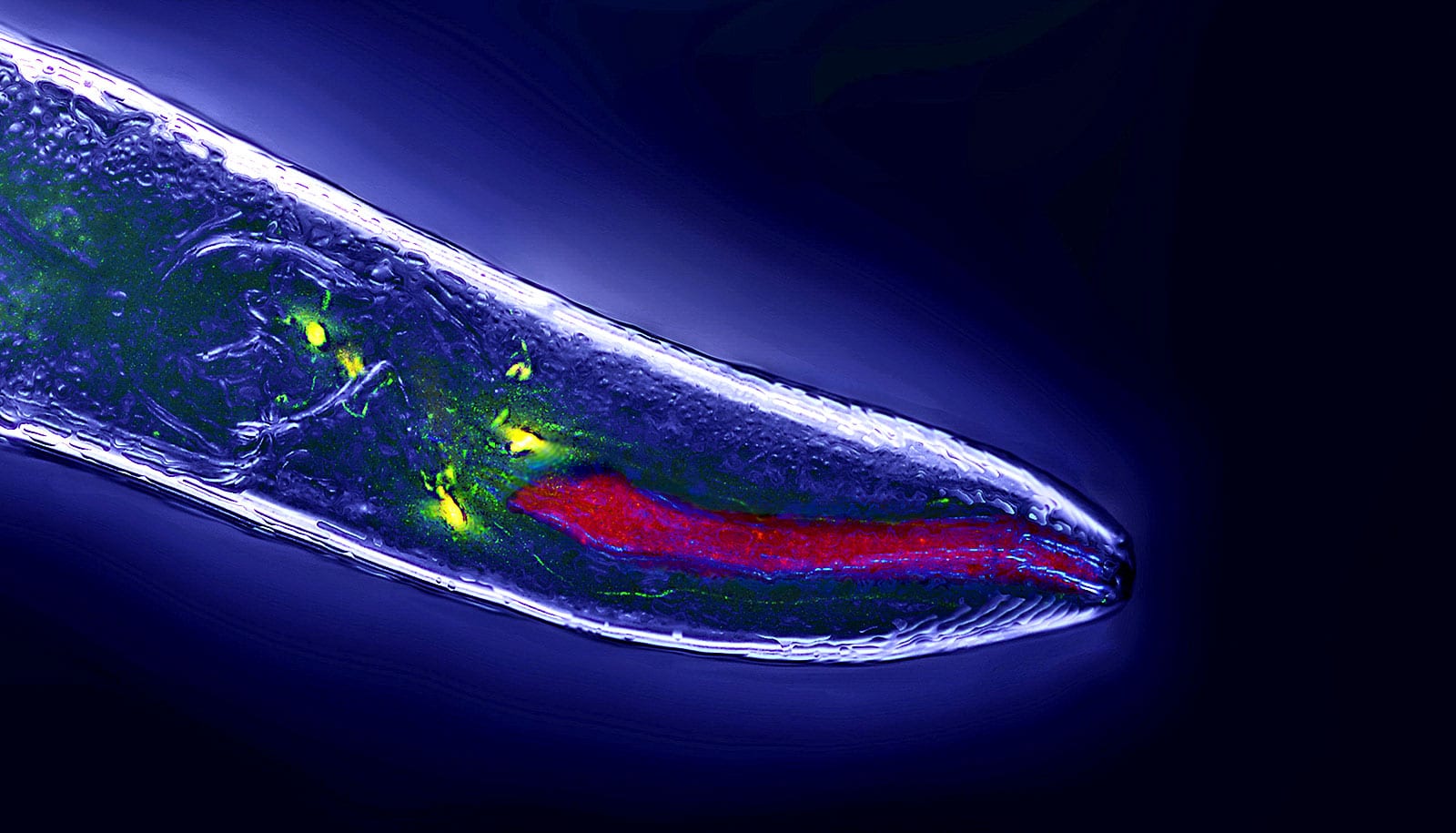
The findings were made in experiments involving the nematode C. elegans, a microscopic roundworm that has long been used by researchers to understand fundamental mechanisms in biology. Many of the discoveries made using these worms apply throughout the animal kingdom and this research has led to a broader understanding of human biology.
The study focuses on the different behaviors of male and female worms. There are two sexes of C. elegans, males and hermaphrodites. Although the hermaphrodites are able to self-fertilize, they are also mating partners for males, and are considered to be modified females.
The behavior of C. elegans is driven by sensory cues, primarily smell and taste, which are used by the worms to navigate their environment and communicate with each other. Female worms secrete a pheromone that is known to attract males who are drawn by this signal in search of a mate. Other females, however, are repelled by the same pheromone. It is not entirely understood why, but scientists speculate that that the pheromone signals to females to avoid areas where there may be too much competition.
The question that scientists sought to answer is why the same stimulus can elicit a different behavioral response based on the sex of the animal. C. elegans are particularly suited to this type of study because scientists understand in great detail the development, function, and multiple connections of its entire neural network.
C. elegans also provides the ideal platform to isolate biological mechanisms of behavior because the results can be observed absent the social influences found in more complex animals.
Same cue, different responses
In the experiment, the scientists are able to manipulate genetic sex of each of the worm’s neurons, essentially switching each individual nerve cell from male to female and vice versa. In doing this, the researchers were able to isolate the sex-specific behavioral differences to a pair of sensory neurons called ADF.
In males, these neurons appear to be “tuned” in a manner that triggers the expression of a gene called mab-3 which signals the worm to follow the pheromone to its source, while in females it generates a different response which instructs the worm to keep its distance. When the biological sex of this single pair of neurons was altered, the males and females flipped their responses to the pheromone.
“These findings demonstrate that the animal’s biological sex instructs the behavioral response,” says Portman. “In this instance, the tuning of a single pair of neurons generates different behaviors in response to a cue that is relevant for both sexes, but one that generates opposite beneficial responses.
“More broadly, this demonstrates mechanisms by which biological sex tweaks and tunes neural networks and could ultimately help us understand why males and females are predisposed to or protected from neurological or neuropsychiatric disorders.”
Additional coauthors are from URMC, Worcester Polytechnic Institute, the Boyce Thompson Institute, and Cornell University. Funding for the study came from the National Institute of General Medicine Sciences, the National Science Foundation, and the Burroughs Wellcome Fund.
Source: University of Rochester