Scientists have developed a new way to prevent the loss of millions of tons of crops to a fungus each year. The work could improve food security, especially in developing countries.
Researchers used transgenic corn plants that produce small RNA molecules that prevent fungi from producing aflatoxin, highly toxic substances that can render an entire harvest unsafe for human consumption even in small amounts.
“Aflatoxin is one of the most potent toxins on the planet. Usually it won’t kill a person outright, but it can make you very sick.”
Although extensive field testing will have to precede widespread application of the new technique in agricultural settings around the world, the results of the study, published in Science Advances, show that transgenic corn plants infected with the fungus suppressed toxin levels below detectable limits.
Crops all over the world are susceptive to infection by fungi of various Aspergillus species, a fungus that produces secondary metabolites known as aflatoxins. These compounds have been implicated in stunting children’s growth, increasing the risk for liver cancer, and making people more susceptible to diseases such as HIV and malaria.
Chocolate-killing fungus skips sex to clone
Unlike in the US, where crops intended for human consumption are tested for aflatoxin and incinerated once levels approach 20 parts per billion (equivalent to one drop of water in a 22,000-gallon pool), no testing is available in many developing parts of the world, especially in Africa, where millions of people depend on consuming what they harvest.
There, toxin levels up to 100,000 parts per billion have been measured, says study leader Monica Schmidt, assistant professor in the University of Arizona’s School of Plant Sciences and a member of the BIO5 Institute.
“Aflatoxin is one of the most potent toxins on the planet,” she says. “Usually it won’t kill a person outright, but it can make you very sick.”
Shut down the toxin
Researchers wanted to find out whether a naturally occurring biological mechanism called RNA interference could be used as a weapon against the Aspergillus toxin. That approach, called Host-Induced Gene Silencing, or HIGS, builds on previous work by other researchers who discovered that during the infectious process, the host plant and the fungus exchange small nucleic acid molecules.
“When I read about this in the literature, I thought, ‘Why can’t we make a Trojan horse to shut off that toxin?'” says Schmidt, who has been working on this project for years. “We introduced an engineered DNA construct into the corn that passes the RNA into the fungus when it infects the corn plant.”
The modified corn plants carry a genetic blueprint for small RNA molecules, each only about 20 base pairs long, only in the edible kernels, not the whole plant.
“The corn is constantly producing that RNA during the entire development of the kernel,” Schmidt explains. “When the kernels come in contact with the fungus, the RNA moves over into the fungus.”
Mold on corn can cause liver cancer
Once inside the fungal cells, the hairpin-shaped RNA molecules pair up with corresponding target sequences of the fungus’ own RNA that code for an enzyme needed for toxin production, in a process called RNA interference. This causes the toxin production to shut down, but does not in any other way impact the fungus, which continues to grow and live on the corn, albeit harmlessly.
Other ways to deal with Aspergillus
The HIGS approach has a distinctive advantage over existing efforts to keep aflatoxin out of the human food chain, because it prevents the fungus from making toxin in the first place while the crop is growing in the field, as opposed to protecting crops only after they have been harvested and stored. Such approaches include solar-powered fans that suck out air from storage facilities or sealing crops in huge storage bags creating airless conditions so fungus cannot grow.
Another strategy, pioneered by study coauthor Peter Cotty, a research plant pathologist with the US Department of Agriculture and the University of Arizona’s School of Plant Sciences, has explored spraying crop plants with strains of Aspergillus that do not produce aflatoxin, thereby preventing their pathogenic kin from establishing themselves on the plants.
Other researchers have tried breeding varieties of corn that express antifungal proteins, but because not many antifungal proteins are known to begin with, those efforts have had limited success, Schmidt says.
HIGS holds great promise because it is highly specific and targeted in its effect, Schmidt explains, and could potentially be applied to other crops as well.
Safe enough to eat
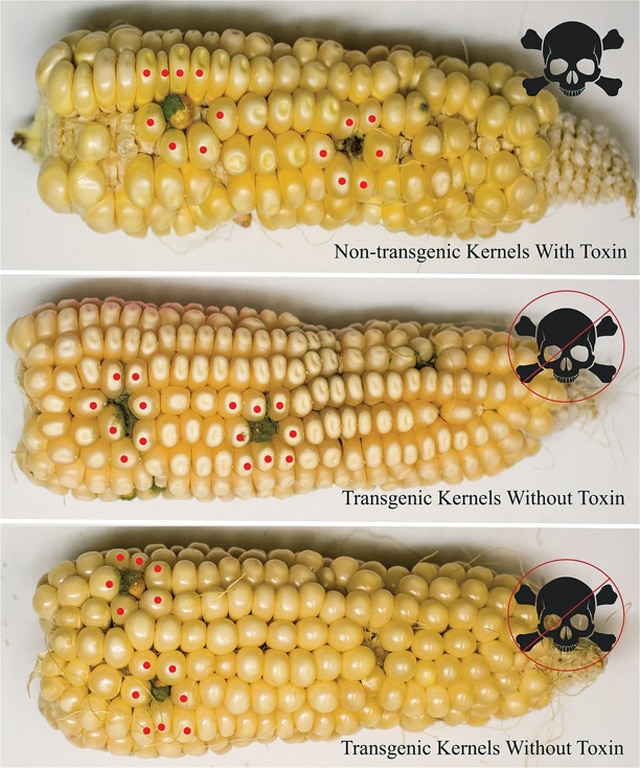
In their experiments, the team infected corn plants with Aspergillus and let them grow for one month. While untreated control plants were found to harbor toxin levels between 1,000 and 10,000 per billion, toxin levels were undetectable in the transgenic plants.
“The detection limit is not zero, but low enough for the corn to be safe to eat,” Schmidt says.
The team took the project a step further and investigated overall gene expression in kernels to see if the transgenic corn plants come with undesired side effects. They compared thousands of RNA transcripts between the nontransgenic control kernels and transgenic kernels. The team did not find a single significant difference in terms of differential gene expression between the transgenic and the non-transgenic kernels, according to Schmidt.
“This corn plant would be like any other,” she says. “The only trait that sets it apart is its ability to shut down the toxin production. It shouldn’t have any other effects, but obviously, a lot of downstream testing will be required before it could be grown in the fields.”
The Bill and Melinda Gates Foundation funded the work. Schmidt is collaborating with the university to bring the technology to the marketplace and a patent has been filed.
Source: University of Arizona



