Scientists have created a durable catalyst for high-performance fuel cells by attaching single ruthenium atoms to graphene.
Catalysts that drive the oxygen reduction reaction that lets fuel cells turn chemical energy into electricity are usually made of platinum, which stands up to the acidic nature of the cell’s charge-carrying electrolyte. But platinum is expensive, and scientists have searched for decades for a suitable replacement.
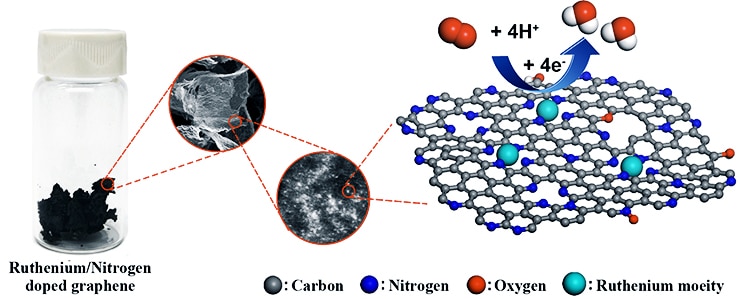
The ruthenium-graphene combination may fit the bill, says chemist James Tour, a professor of computer science and of materials science and nanoengineering at Rice University, whose lab developed the material. In tests, its performance easily matched that of traditional platinum-based alloys and bested iron and nitrogen-doped graphene, another contender.
“Ruthenium is often a highly active catalyst when fixed between arrays of four nitrogen atoms, yet it is one-tenth the cost of traditional platinum,” Tour says. “And since we are using single atomic sites rather than small particles, there are no buried atoms that cannot react. All the atoms are available for reaction.”
Here’s a cheaper way to make a graphene catalyst
Spreading single ruthenium atoms across a sheet of graphene, the atom-thick form of carbon, turned out to be fairly straightforward, Tour says. It involved dispersing graphene oxide in a solution, loading in a small amount of ruthenium, and then freeze-drying the new solution and turning it into a foam.
Baking that at 750 degrees Celsius (1,382 degrees Fahrenheit) in the presence of nitrogen and hydrogen gas reduced the graphene and locked nitrogen atoms to the surface, providing sites where ruthenium atoms could bind.
Materials made at higher and lower temperatures weren’t as good, and those made at the proper temperature but without either ruthenium or nitrogen proved the quality of the reaction depended on the presence of both.
The material showed excellent tolerance against methanol crossover and carbon monoxide poisoning in an acidic medium, both of which degrade the efficiency of fuel cells; such degradation is a persistent problem with traditional platinum fuel cells.
A paper on the discovery appears in the journal ACS Nano.
0.01-nanometer squeeze improves platinum catalysts
Additional authors of the paper are from Rice University; the Chinese Academy of Sciences, Shanghai; Tianjin University; and the Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China.
The Air Force Office of Scientific Research and its Multidisciplinary University Research Initiative; the China Scholarship Council; the American Chemical Society Petroleum Research Fund; the Department of Energy; the Robert Welch Foundation; the National Natural Science Foundation of China; and the Jianlin Xie Foundation of the Institute of High Energy Physics, Chinese Academy of Science supported the research.
Source: Rice University



