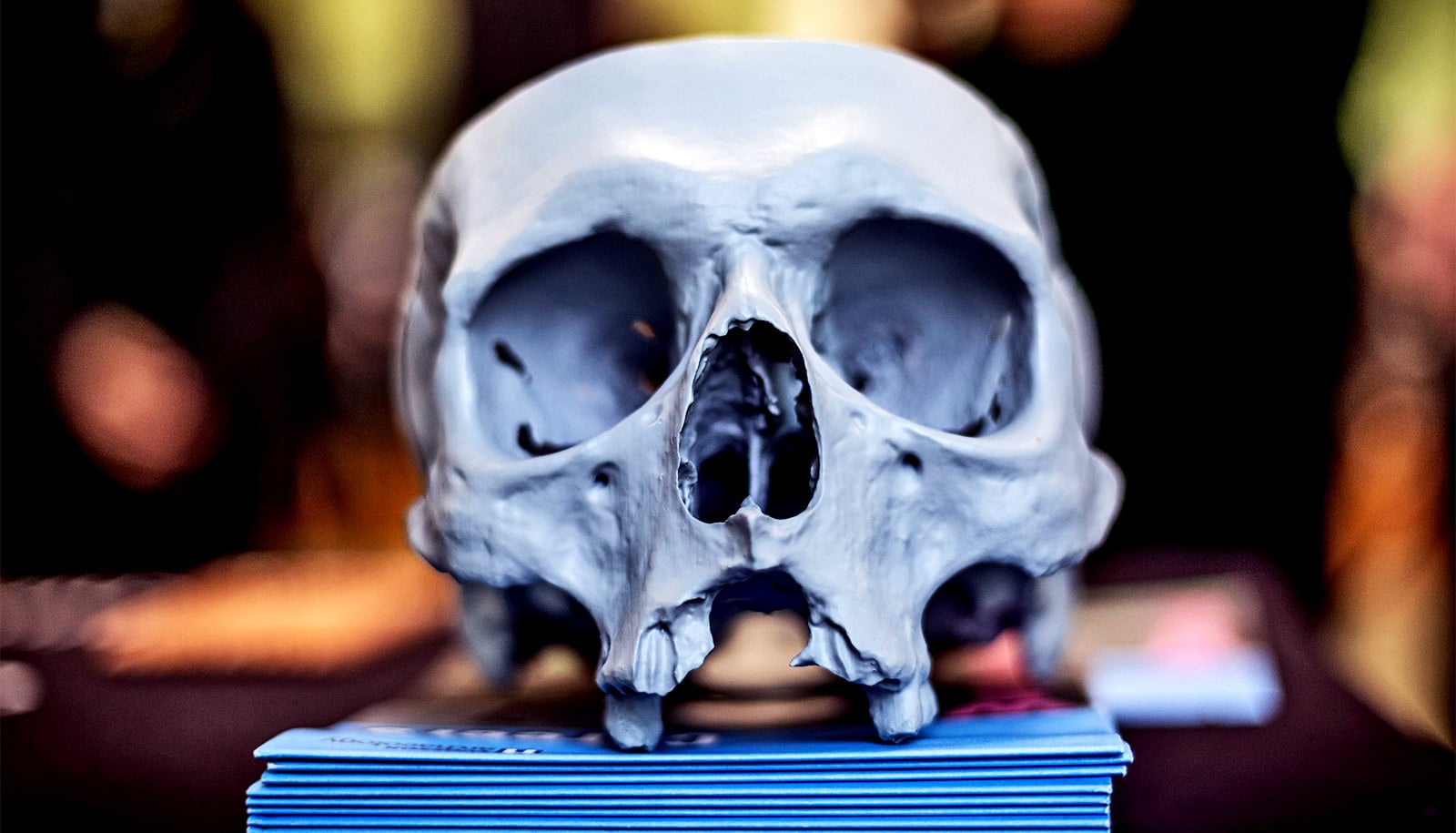
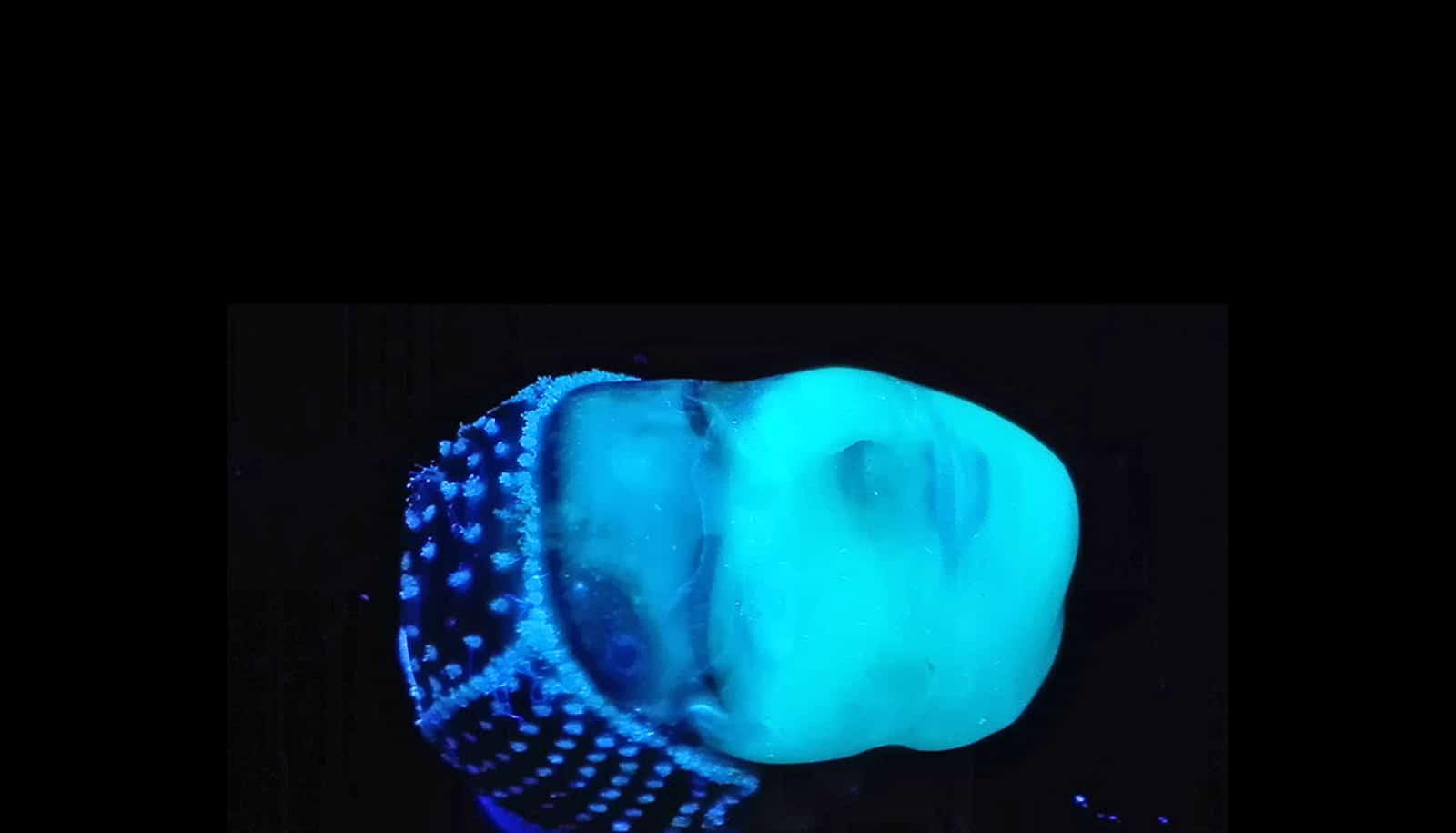
Bioprinting is an emerging additive manufacturing approach that takes biomaterials such as hydrogels and combines them with cells and growth factors, which researchers then print to create tissue-like structures that imitate natural tissues.
One application of this technology could be designing patient-specific bone grafts, an area that is gaining interest from researchers and clinicians.
Managing bone defects and injuries through traditional treatments tends to be slow and expensive. Gaharwar says that developing replacement bone tissues could create exciting new treatments for patients suffering from arthritis, bone fractures, dental infections, and craniofacial defects.
Bioprinting requires cell-laden biomaterials that can flow through a nozzle like a liquid, but solidify almost as soon as they’re deposited. These bioinks need to act as both cell carriers and structural components, requiring them to be highly printable while providing a robust and cell-friendly microenvironment.
However, current bioinks lack sufficient biocompatibility, printability, structural stability, and tissue-specific functions needed to translate this technology to preclinical and clinal applications.
To address this issue, the researchers are leading efforts in developing advanced bioinks known as Nanoengineered Ionic-Covalent Entanglement (NICE) bioinks. NICE bioinks are a combination of two reinforcement techniques (nonreinforcement and ionic-covalent network), which together provide more effective reinforcement that results in much stronger structures.
Once bioprinting is complete, the researchers crosslink the cell-laden NICE networks to form stronger scaffolds. This technique has allowed the lab to produce full-scale, cell-friendly reconstructions of human body parts, including ears, blood vessels, cartilage, and even bone segments.
Soon after the bioprinting, the enclosed cells start depositing new proteins rich in a cartilage-like extracellular matrix that subsequently calcifies to form a mineralized bone over a three-month period. Almost 5% of these printed scaffolds consisted of calcium, which is similar to cancellous bone, the network of spongy tissue typically found in vertebral bones.
To understand how these bioprinted structures induce stem cell differentiation, the researchers used a next-generation genomics technique called whole transcriptome sequencing (RNA-seq). RNA-seq takes a snapshot of all genetic communication inside the cell at given moment.
“The next milestone in 3D bioprinting is the maturation of bioprinted constructs toward the generation of functional tissues,” Gaharwar says. “Our study demonstrates that NICE bioink developed in our lab can be used to engineer 3D-functional bone tissues.”
In the future, Gaharwar’s team plans to demonstrate in vivo functionality of the 3D-bioprinted bone tissue.
The research appears in Applied Materials and Interfaces.
Funding for the research came from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health’s Director’s New Innovator Award, a National Science Foundation’s Award, and an X-Grant from Texas A&M University.
Source: Texas A&M University