New research reveals why a tiny tab of acid on the tongue can set off a daylong trip through hallucinations and other psychedelic experiences.
The work shows precisely what the drug lysergic acid diethylamide (LSD) looks like in its active state when attached to a human serotonin receptor of a brain cell, and the first-ever crystal structure reveals a major clue for why the psychoactive effects of LSD last so long.
“There are different levels of understanding for how drugs like LSD work,” says Bryan L. Roth, professor of protein therapeutics and translational proteomics in the UNC School of Medicine. “The most fundamental level is to find out how the drug binds to a receptor on a cell. The only way to do that is to solve the structure. And to do that, you need X-ray crystallography, the gold standard.”
That is what Roth’s lab accomplished—essentially “freezing” LSD attached to a receptor so his team could capture crystallography images. As it turns out, when LSD latches onto a brain cell’s serotonin receptor, the LSD molecule is locked into place because part of the receptor folds over the drug molecule, like a lid. And then it stays put.
How awe could boost your health
“We think this lid is likely why the effects of LSD can last so long,” says Roth, who holds a joint appointment at the UNC Eshelman School of Pharmacy. “LSD takes a long time to get onto the receptor, and then once it’s on, it doesn’t come off. And the reason is this lid.”
Eventually, though, an acid trip ends. Some LSD molecules pop off their receptors as the lid moves around. Also, brain cells eventually respond to this strange molecule by sucking the receptor into the cell, where it—along with the LSD—is degraded or disassembled for recycling.
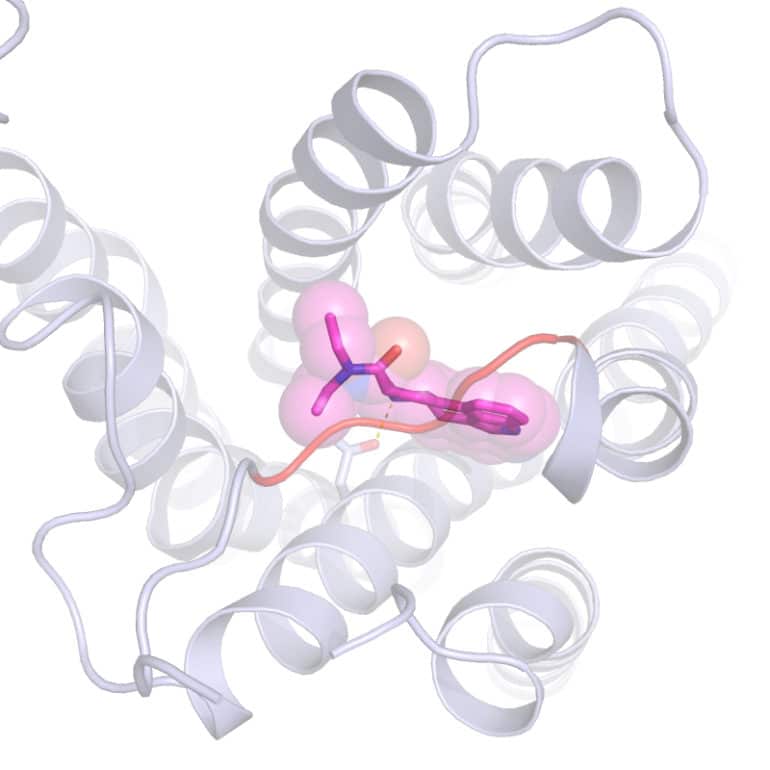
Postdoctoral researchers Daniel Wacker and Sheng Wang led the experiments, reported in the journal Cell, to crystallize LSD bound to a serotonin receptor and discover why it stays bound so long. “Serotonin, obviously, hits this receptor on brain cells,” Wacker says. “But our experiments show that serotonin does not interact with this lid in the same way LSD does.”
Although other labs have reported that LSD “washes” out of the brain’s fluid within four hours, such experiments could not determine what was happening on or inside brain cells. Roth’s lab has shown for the first time that LSD is very much not washed out of the serotonin receptors located within the membrane of brain cells in a few hours.
New uses and ‘microdosing’
How this popular drug causes such powerful effects has remained a mystery ever since Swiss scientist Albert Hofmann first accidently synthesized and dosed LSD to report its effects in 1938. Now, because of the work of Roth’s lab, scientists can begin to parse how the drug sparks such a dramatic reaction in the brain, just as the scientific and medical communities renew interest in the drug as a potential treatment for a number of conditions, such as cluster headaches, substance abuse, and anxiety associated with life-threatening conditions.
‘Magic mushroom’ drug eases cancer patient anxiety
Solving the structure of LSD could help drug developers design better psychiatric drugs with fewer side effects. Also, although LSD is illegal, it remains a popular recreational drug and not just for its most potent effects. Some people—most notably technology developers in Silicon Valley and elsewhere—report “microdosing” LSD to boost creativity, relieve stress, and help them solve problems, while avoiding its hallucinogenic effects.
One in 10 people in the United States—tens of millions of people—have reported using LSD at least once in their lives. “About 3 percent of all high school students—who are at an age when their brains are still developing—have reported trying it,” Roth says. “And although the drug has been used for a long time, we don’t know that much about it.”
Previous to becoming a pharmacology professor and researcher, Roth was a psychiatrist specializing in schizophrenia. Patients would occasionally report that their first schizophrenic break occurred while on LSD.
“They were never the same again,” Roth says. “Although this is rare, it has been reported. People also report flashbacks and LSD is an extremely potent drug. So for those reasons, along with its potential as part of therapeutic treatment, LSD is scientifically interesting.”
‘A dynamic connection’
Ron Dror and his team at Stanford University used computer simulations to confirm that this is what might happen when LSD engages its receptor protein in a human brain.
“There is a headache drug that binds to the same receptor as LSD,” Dror says. “The two drugs bind in the same receptor pocket, but the shape of that binding pocket is different when one drug or the other is bound. We used computer simulations to help explain why the two drugs favor different binding pocket shapes.
Another aspect of this computational work focused on the fact that the receptor site is not static—the receptor and the drug are both highly dynamic. “They wiggle around all the time, Dror says. “It has long been observed that LSD trips are long. The simulations helped explain why the receptor holds onto LSD for so long despite the fact that they have such a dynamic connection.”
Roth says, “We do not advocate using LSD; it is potentially very dangerous. But it could have potential medicinal uses, some of which were reported in the medical literature decades ago. Now that we’ve solved the structure of LSD bound to a receptor, we are learning what makes it so potent.”
Additional coauthors from UNC, Stanford, and UC San Francisco contributed to the work, which the National Institute of Mental Health, a Terman Faculty Fellowship, and the Michael Hooker Distinguished Chair of Pharmacology at UNC funded.
Source: UNC-Chapel Hill



